The boosters contain protection for both the original strain of COVID-19 and the Omicron variant. And now, they're available for the youngest vaccine-eligible population.
“More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to consider doing so—especially as we head into the holidays and winter months where more time will be spent indoors,” said FDA Commissioner Robert M. Califf.
Children that haven’t begun their three-dose primary series of the Pfizer-BioNTech COVID-19 vaccine or haven’t gotten the third dose of their primary series, will now get the bivalent Pfizer-BioNTech vaccine as that third booster dose after two doses of the original vaccine. Children who have already completed their three-dose primary series with the original Pfizer-BioNTech COVID-19 vaccine are not currently eligible for a booster dose of an updated bivalent vaccine at this time. The Fthat children in this age group who have already completed their primary vaccination series still have strong protection against the most serious outcomes of the virus, even from the Omicron variant.
“Vaccines remain the best defense against the most devastating consequences of disease caused by the currently circulating omicron variant, such as hospitalization and death. Based on available data, the updated, bivalent vaccines are expected to provide increased protection against COVID-19,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research,.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
Weiterlesen »
 Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Weiterlesen »
 Children as young as 6 months can now receive an updated Covid-19 vaccine | CNNThe US Food and Drug Administration on Thursday authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Children as young as 6 months can now receive an updated Covid-19 vaccine | CNNThe US Food and Drug Administration on Thursday authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Weiterlesen »
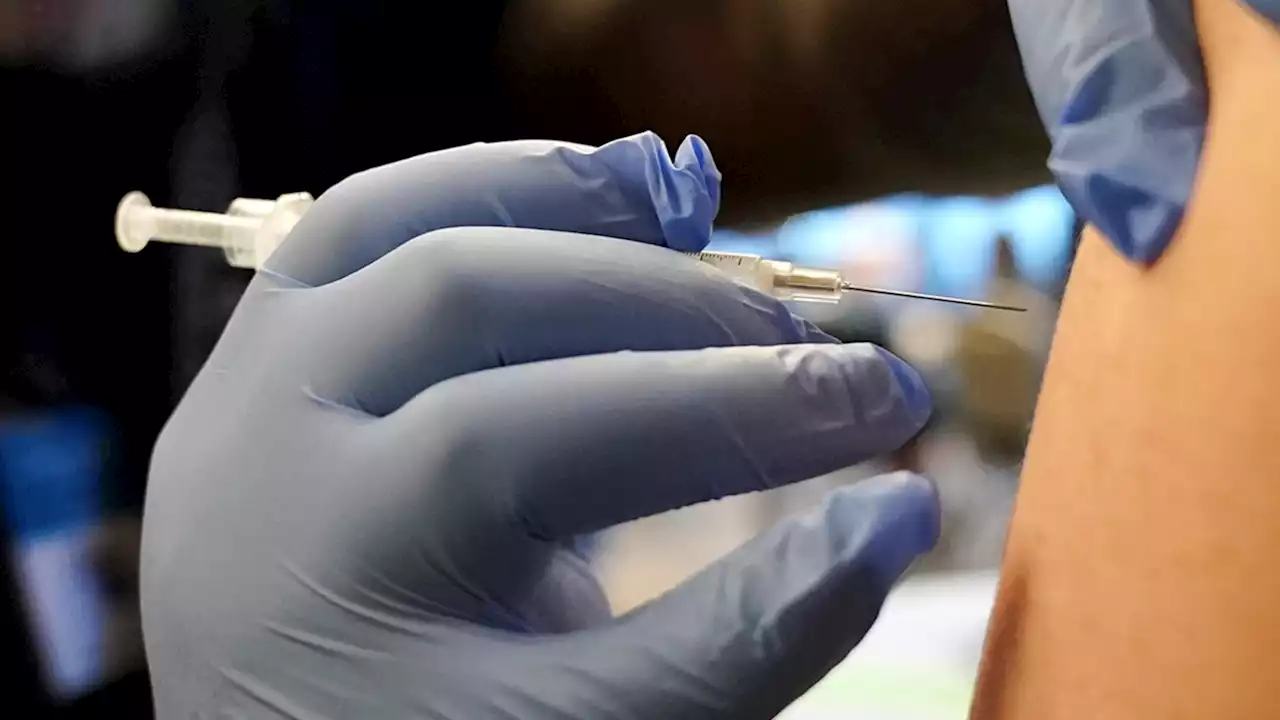 Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Weiterlesen »
 Some children as young as 6 months now eligible for COVID-19 booster shotsAlthough the updated boosters have been available since September, only a small percentage of Americans have gotten one.
Some children as young as 6 months now eligible for COVID-19 booster shotsAlthough the updated boosters have been available since September, only a small percentage of Americans have gotten one.
Weiterlesen »