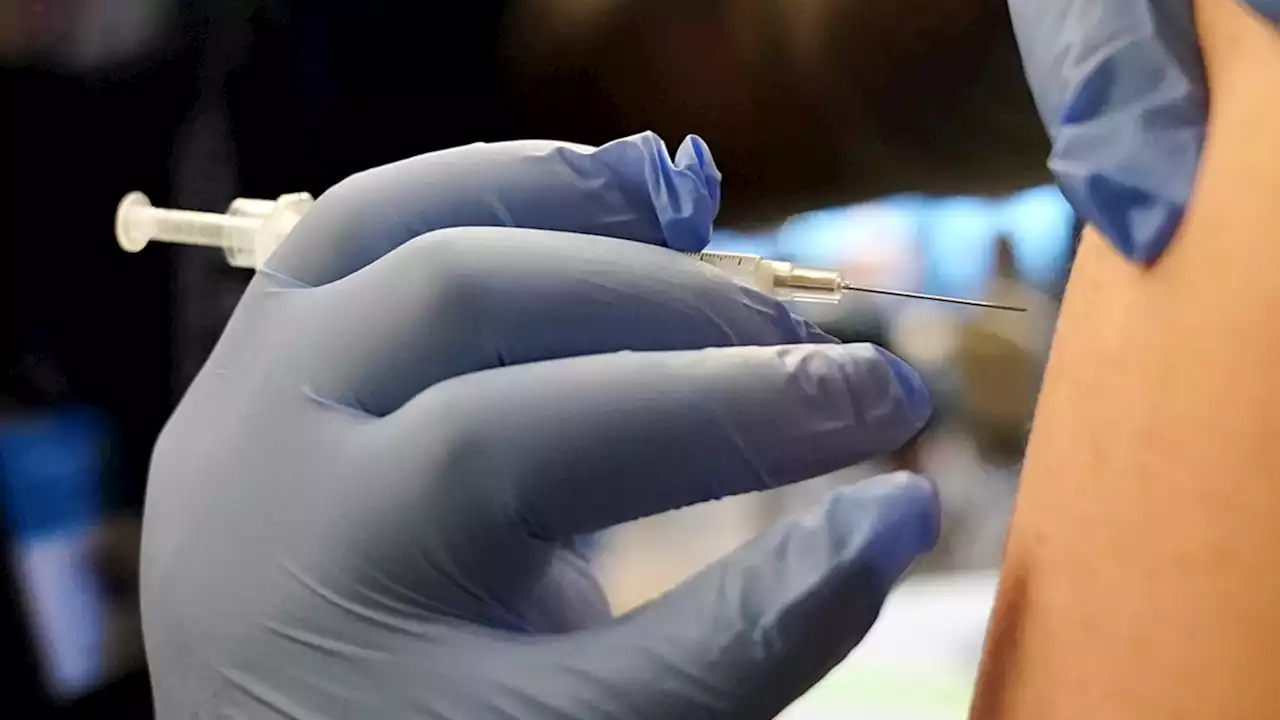
Children as young as 6 months are now eligible to receive an updated Covid-19 vaccine.
The director of the Centers for Disease Control participated as a speaker at the Bloomberg American Health Summit in Center City Philadelphia.
The bivalent vaccines target the original strain as well as the BA.4/5 Omicron strains. Bivalent vaccines were previously authorized as a booster for people age 5 and older. Children age 6 months through 4 years who haven't started their three-dose primary series of the Pfizer vaccine or received the third dose will receive the updated Pfizer vaccine as the third dose of the primary series.
"Children in this age group who already completed their primary series would still be expected to have protection against the most serious outcomes from the currently circulating omicron variant. The data to support giving an updated bivalent booster dose for these children are expected in January. The agency is committed to evaluating those data as quickly as possible," the FDA said in its announcement.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
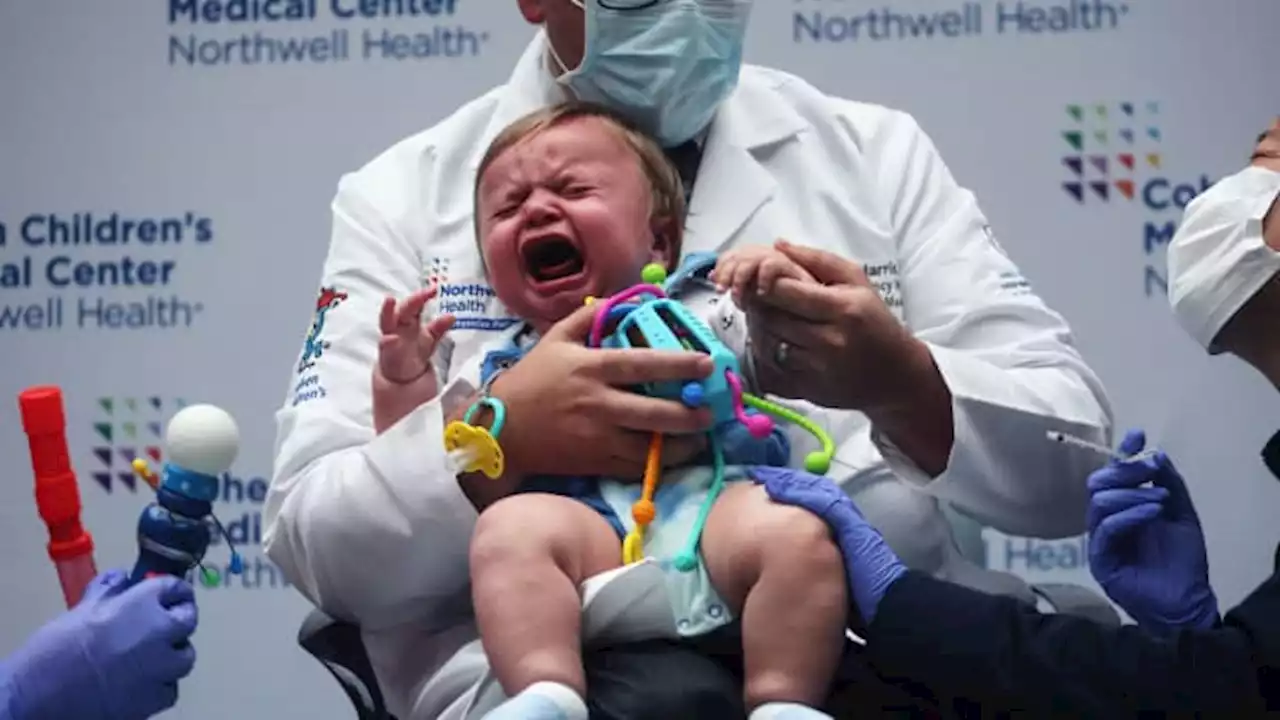 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
Weiterlesen »
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
Weiterlesen »
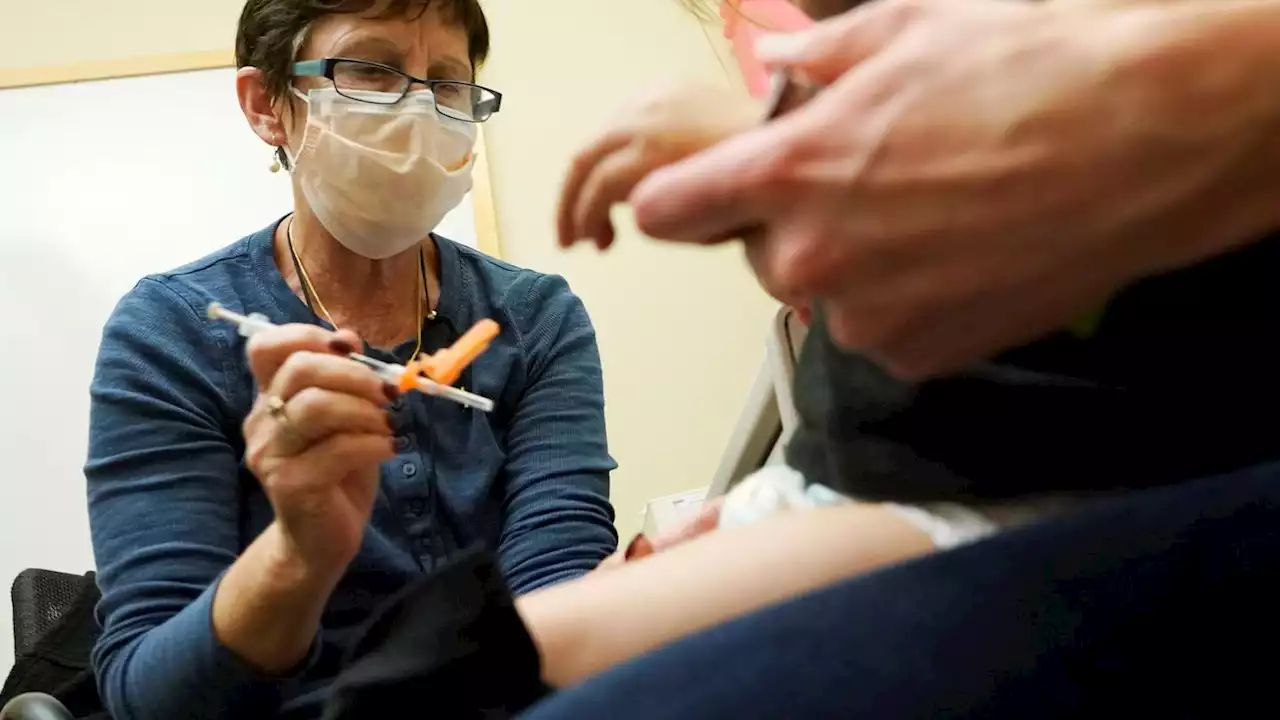 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
Weiterlesen »
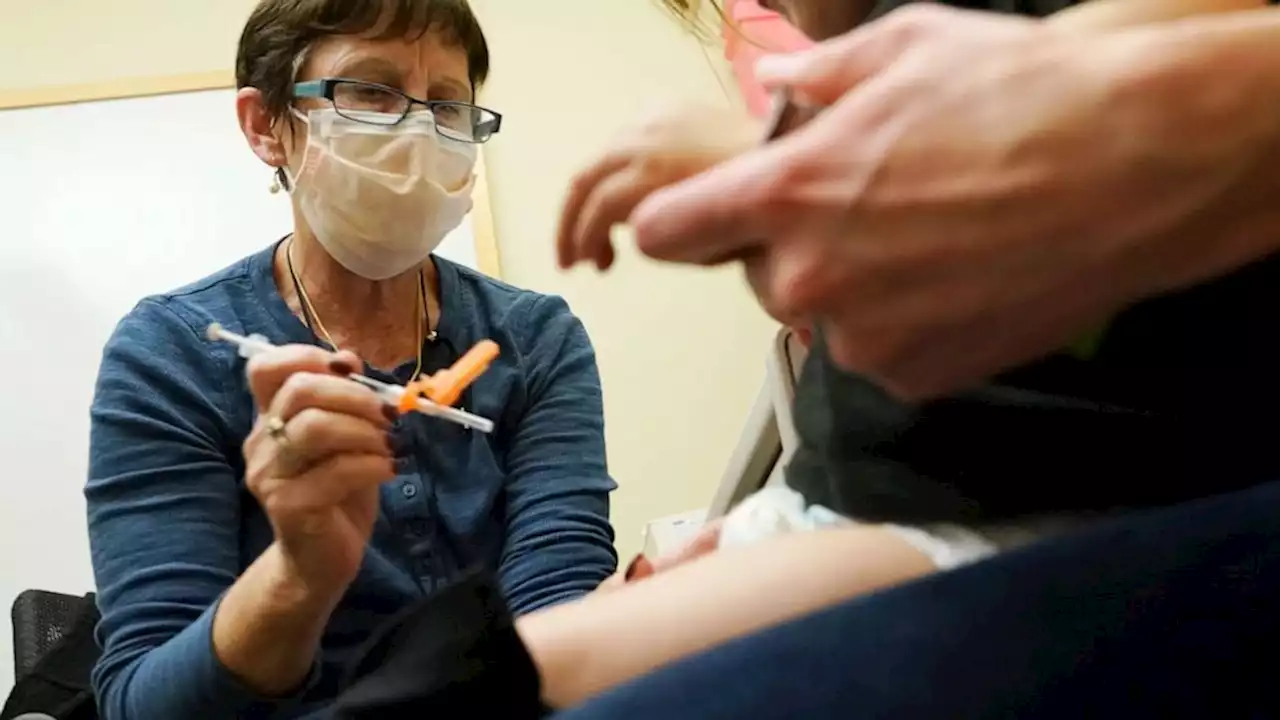 FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
Weiterlesen »
 NJ COVID hospitalizations hit 10-month highThe state is currently seeing the highest number of COVID-19 hospitalizations since February.
NJ COVID hospitalizations hit 10-month highThe state is currently seeing the highest number of COVID-19 hospitalizations since February.
Weiterlesen »