At CES this year, Withings announced its Body Scan Connected Health Station. It’s arguably the most extra smart scale to exist with six-lead EKG and atrial fibrillation detection, segmented body composition, electrodermal activity sensors to detect nerve health from your feet sweat, and the ability to estimate your vascular age. Now that the FDA’s cleared the product, Withings says it’ll go on sale this fall for a whopping $399.95. Would you use this scale?
At CES this year, Withings announced its Body Scan Connected Health Station. It’s arguably the most extra smart scale to exist with six-lead EKG and atrial fibrillation detection, segmented body composition, electrodermal activity sensors to detect nerve health from your feet sweat, and the ability to estimate your vascular age.
Now that the FDA’s cleared the product, Withings says it’ll go on sale this fall for a whopping $399.95. Would you use this scale?
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
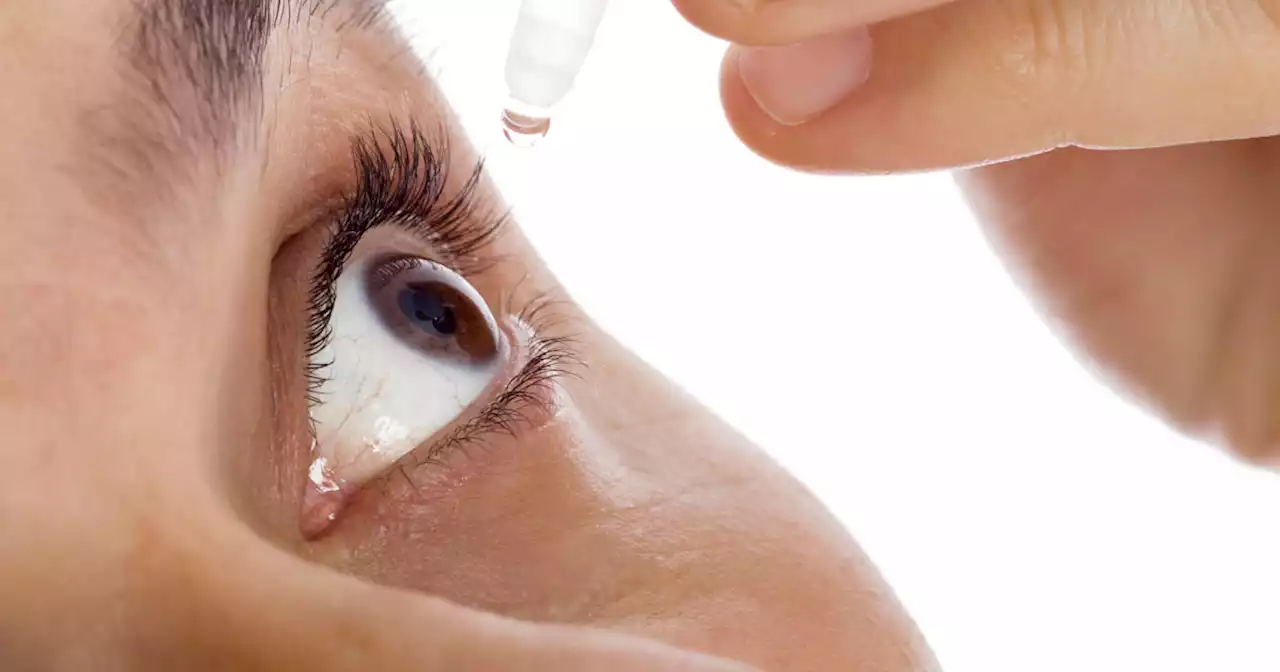 FDA says to stop using 2 eye drop products because of serious health risksUsing eye drops contaminated with bacteria can lead to a 'life-threatening infection,' FDA warns.
FDA says to stop using 2 eye drop products because of serious health risksUsing eye drops contaminated with bacteria can lead to a 'life-threatening infection,' FDA warns.
Weiterlesen »
 US FDA panel votes against use of Medtronic's blood pressure treatment device By ReutersUS FDA panel votes against use of Medtronic's blood pressure treatment device
US FDA panel votes against use of Medtronic's blood pressure treatment device By ReutersUS FDA panel votes against use of Medtronic's blood pressure treatment device
Weiterlesen »
 New warning from FDA against using these 2 eyedropsThe drops are sold online, but are considered illegal because they contain methylsulfonylmethane, or MSM, an ingredient that is not approved for use in the U.S. and illegally marketed
New warning from FDA against using these 2 eyedropsThe drops are sold online, but are considered illegal because they contain methylsulfonylmethane, or MSM, an ingredient that is not approved for use in the U.S. and illegally marketed
Weiterlesen »
 Novartis's Sandoz Gets FDA Approval for Multiple Sclerosis BiosimilarBy Adria Calatayud Novartis's Sandoz generic-drugs unit has received approval from the U.S. Food and Drug Administration for biosimilar medicine Tyruko to...
Novartis's Sandoz Gets FDA Approval for Multiple Sclerosis BiosimilarBy Adria Calatayud Novartis's Sandoz generic-drugs unit has received approval from the U.S. Food and Drug Administration for biosimilar medicine Tyruko to...
Weiterlesen »
 Medtronic shares fall after FDA advisors’ lukewarm vote on blood-pressure treatment deviceMedtronic PLC shares were down 1.3% premarket on Thursday after advisors to the U.S. Food and Drug Administration had a mixed reaction to the company’s...
Medtronic shares fall after FDA advisors’ lukewarm vote on blood-pressure treatment deviceMedtronic PLC shares were down 1.3% premarket on Thursday after advisors to the U.S. Food and Drug Administration had a mixed reaction to the company’s...
Weiterlesen »
 FDA issues alert on 2 eye drops that tested positive for contaminantsUsers of two types of eye drops are being warned to stop using them because the FDA said they may contain bacterial and fungal contamination.
FDA issues alert on 2 eye drops that tested positive for contaminantsUsers of two types of eye drops are being warned to stop using them because the FDA said they may contain bacterial and fungal contamination.
Weiterlesen »
