A new milestone for a fatal disease that has eluded drugmakers' efforts for decades.
Leqembi won a coveted standard approval nod from the U.S. Food and Drug Administration on Thursday, the first Alzheimer's treatment to achieve that goal, clearing the way for wider insurance coverage of the drug.
Shares of Eisai tumbled in Tokyo trade on Friday, with analysts citing the safety warning as a negative surprise. Leqembi received "accelerated" FDA approval in January based on its amyloid-clearing ability, but the U.S. government's Medicare health plan for people aged 65 and over had restricted coverage only to patients in a clinical trial.
Tousi said Leqembi "is not a cure," and will not turn back time on the disease, it will just slow progression. "This is just one piece of the puzzle," he said. "The boxed warning seems appropriate, as the risks need to be carefully considered and discussed with patients," Dr. Erik Musiek, a Washington University neurologist at Barnes-Jewish Hospital, said in an email.Analysts at Jefferies said the FDA's unexpected decision to put the warning on the label could contribute to slow sales growth for the drug, noting that the recommendation for genetic testing would increase costs for prospective patients.
Cheung said Eisai is expanding efforts to get health centers ready to use Leqembi, but declined to comment on how many patients have been treated with the drug so far.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA to grant full approval for Alzheimer's medication LecanemabIf the FDA grants Lecanemab full approval, medical experts say Medicaid and Medicare will likely cover it MORE ⬇️
FDA to grant full approval for Alzheimer's medication LecanemabIf the FDA grants Lecanemab full approval, medical experts say Medicaid and Medicare will likely cover it MORE ⬇️
Weiterlesen »
 FDA approves use of Leqembi to treat Alzheimer's patientsFDA approves use of Leqembi Alzheimer\u0027s patients
FDA approves use of Leqembi to treat Alzheimer's patientsFDA approves use of Leqembi Alzheimer\u0027s patients
Weiterlesen »
 Japanese pharma Eisai slides 4% despite FDA approval for Alzheimer's drugLeqembi is the first Alzheimer's antibody treatment to receive full FDA approval.
Japanese pharma Eisai slides 4% despite FDA approval for Alzheimer's drugLeqembi is the first Alzheimer's antibody treatment to receive full FDA approval.
Weiterlesen »
 FDA to decide on Alzheimer’s drug approvalFull approval would likely prompt the Centers for Medicare and Medicaid Services to change how it covers the drug, broadening access for up to about a million people with early onset Alzheimer’s.
FDA to decide on Alzheimer’s drug approvalFull approval would likely prompt the Centers for Medicare and Medicaid Services to change how it covers the drug, broadening access for up to about a million people with early onset Alzheimer’s.
Weiterlesen »
 FDA to decide on Alzheimer’s drug approvalFull approval would likely prompt the Centers for Medicare and Medicaid Services to change how it covers the drug, broadening access for up to about a million people with early onset Alzheimer’s.
FDA to decide on Alzheimer’s drug approvalFull approval would likely prompt the Centers for Medicare and Medicaid Services to change how it covers the drug, broadening access for up to about a million people with early onset Alzheimer’s.
Weiterlesen »
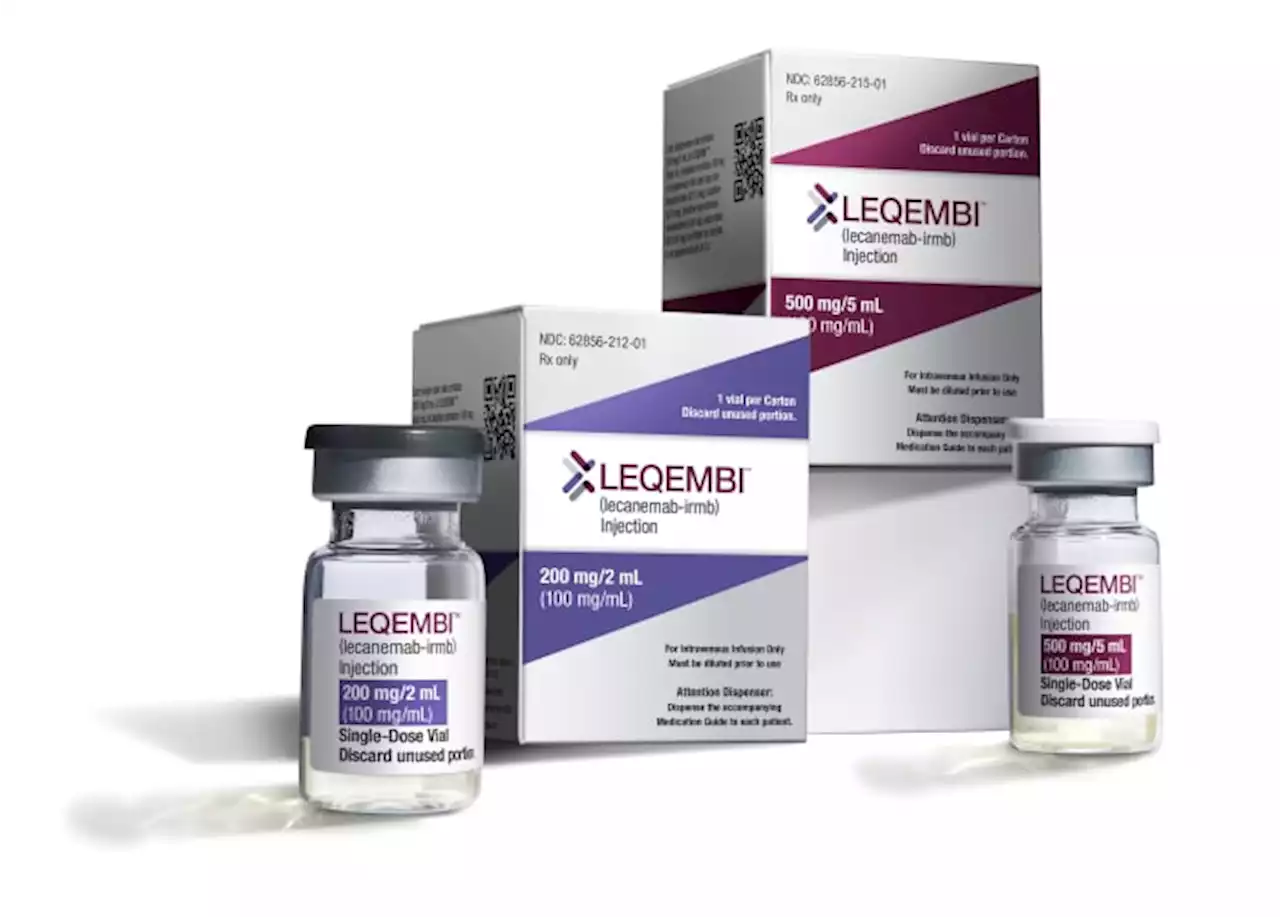 FDA to make a decision on whether to approve first Alzheimer’s drugThe FDA is set to decide whether it will fully approve the first Alzheimer’s drug to show it could slow the disease’s progression in certain patients. But the decision could also have other implications, including who would get access to it.
FDA to make a decision on whether to approve first Alzheimer’s drugThe FDA is set to decide whether it will fully approve the first Alzheimer’s drug to show it could slow the disease’s progression in certain patients. But the decision could also have other implications, including who would get access to it.
Weiterlesen »
