Tetravalent COVID vaccine provides broad antibody responses against SARS-CoV-2 variants in animal model AnimalModel Antibody SARSCoV2 Vaccine Coronavirus Disease COVID biorxivpreprint PittTweet unipv
By Pooja Toshniwal PahariaMar 21 2023Reviewed by Danielle Ellis, B.Sc. *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background Antibodies eBook Compilation of the top interviews, articles, and news in the last year. Download a free copy Coronavirus disease 2019 vaccines have effectively reduced morbidity and mortality associated with SARS-CoV-2 infections. However, the continual emergence of increasingly transmissible and immune-evasive VOCs has threatened vaccine efficacy. Therefore, updated multivalent vaccines conferring durable and broad immune protection are required to mitigate COVID-19.
About the study In the present study, researchers evaluate the immunogenicity of a tetravalent SARS-CoV-2 S1 vaccine, targeting Wuhan-Hu-1, Alpha, Beta, and Gamma S1, among RMs; infected with SIV. Subsequently, the proteins were purified and transiently expressed in Expi293 cells. Transfection experiments were performed, following which the supernatants were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analyses. Rhesus macaques peripheral blood mononuclear cells were obtained on days 1.0, 3.0, 7.0, 10.0, 14.0., 21.0, 24.0, 28.0, 31.0, 35.0, 42.0, 49.0, and 64.0 days after prime vaccination.
Results The tetravalent vaccine approach induced cellular and humoral immunological responses, with B- and T-lymphocyte responses peaking mainly after booster vaccinations. In addition, the tetravalent vaccine induced elicited cross-reactive and neutralizing antibodies, Angiotensin-converting enzyme 2 – blocking antibodies , and cell-mediated responses, including S-targeted CD4+ T lymphocytes.
Decreased abundance of helper and central cytotoxic memory T lymphocytes, helper T, and cytotoxic T-naive T lymphocytes were observed following prime vaccination and booster vaccination.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 Identification of a conserved S2 epitope present on spike proteins from all highly pathogenic coronavirusesA new class of antibody binding a highly conserved epitope on the spike S2 domain of MERS-CoV, SARS-CoV, and SARS-CoV-2 viruses is described.
Identification of a conserved S2 epitope present on spike proteins from all highly pathogenic coronavirusesA new class of antibody binding a highly conserved epitope on the spike S2 domain of MERS-CoV, SARS-CoV, and SARS-CoV-2 viruses is described.
Weiterlesen »
 Control of SARS-CoV-2 infection by MT1-MMP-mediated shedding of ACE2 - Nature CommunicationsThe role of soluble angiotensin converting enzyme 2 (sACE2) in SARS-CoV-2 infection is not well understood. Here, authors show that membrane type 1 matrix metalloproteinase (MT1-MMP) releases sACE2 to promote SARS-CoV-2 cell entry in vitro and in vivo, and the upregulation of MT1-MMP may contribute to increased susceptibility to SARS-CoV-2 infection in ageing.
Control of SARS-CoV-2 infection by MT1-MMP-mediated shedding of ACE2 - Nature CommunicationsThe role of soluble angiotensin converting enzyme 2 (sACE2) in SARS-CoV-2 infection is not well understood. Here, authors show that membrane type 1 matrix metalloproteinase (MT1-MMP) releases sACE2 to promote SARS-CoV-2 cell entry in vitro and in vivo, and the upregulation of MT1-MMP may contribute to increased susceptibility to SARS-CoV-2 infection in ageing.
Weiterlesen »
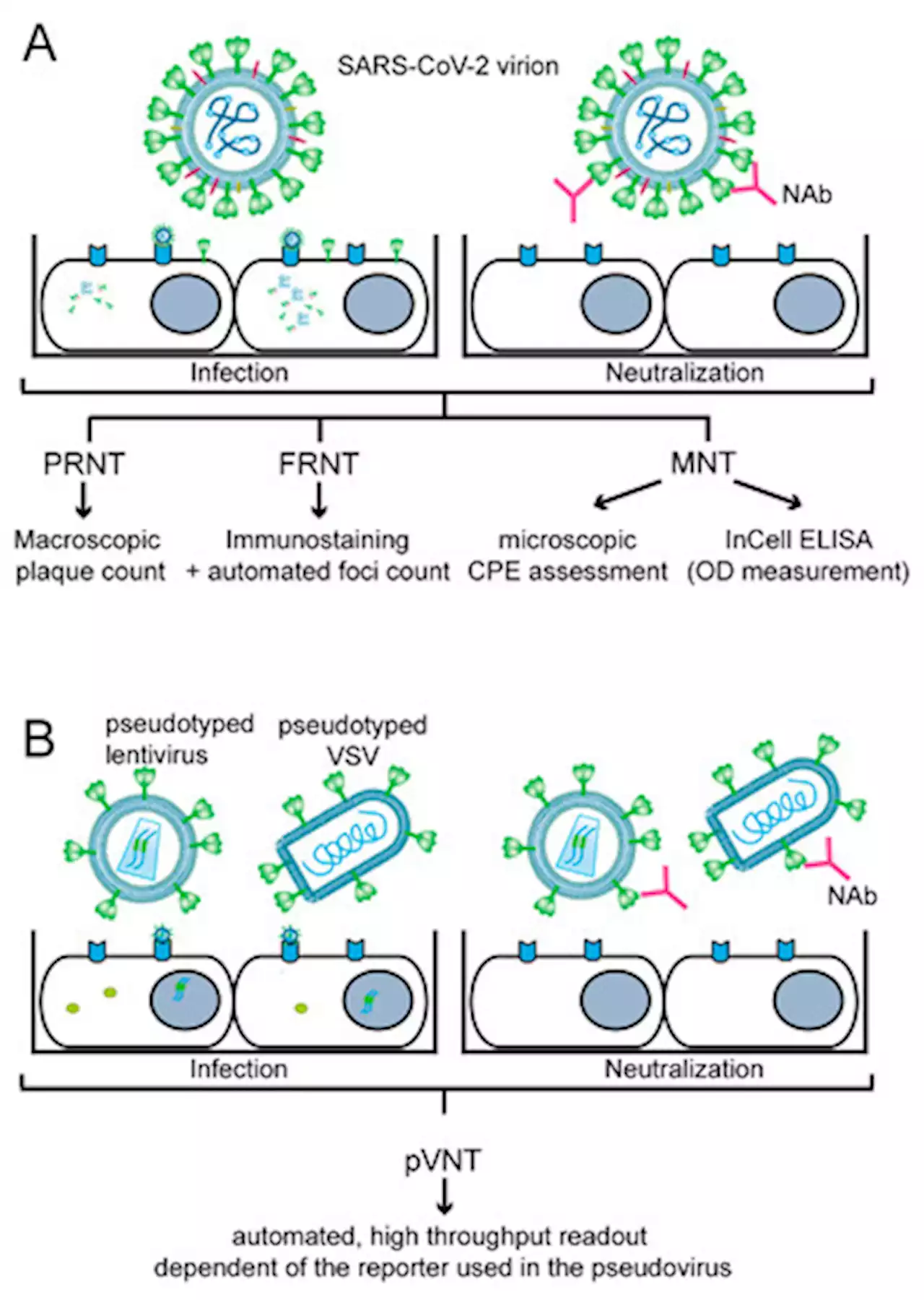 Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing AntibodiesMore than three years ago, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) caused the unforeseen COVID-19 pandemic with millions of deaths. In the meantime, SARS-CoV-2 has become endemic and is now part of the repertoire of viruses causing seasonal severe respiratory infections. Due to several factors, among them the development of SARS-CoV-2 immunity through natural infection, vaccination and the current dominance of seemingly less pathogenic strains belonging to the omicron lineage, the COVID-19 situation has stabilized. However, several challenges remain and the possible new occurrence of highly pathogenic variants remains a threat. Here we review the development, features and importance of assays measuring SARS-CoV-2 neutralizing antibodies (NAbs). In particular we focus on in vitro infection assays and molecular interaction assays studying the binding of the receptor binding domain (RBD) with its cognate cellular receptor ACE2. These assays, but not the measurement of SARS-CoV-2-specific antibodies per se, can inform us of whether antibodies produced by convalescent or vaccinated subjects may protect against the infection and thus have the potential to predict the risk of becoming newly infected. This information is extremely important given the fact that a considerable number of subjects, in particular vulnerable persons, respond poorly to the vaccination with the production of neutralizing antibodies. Furthermore, these assays allow to determine and evaluate the virus-neutralizing capacity of antibodies induced by vaccines and administration of plasma-, immunoglobulin preparations, monoclonal antibodies, ACE2 variants or synthetic compounds to be used for therapy of COVID-19 and assist in the preclinical evaluation of vaccines. Both types of assays can be relatively quickly adapted to newly emerging virus variants to inform us about the magnitude of cross-neutralization, which may even allow us to estimate the risk of becoming infected by new
Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing AntibodiesMore than three years ago, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) caused the unforeseen COVID-19 pandemic with millions of deaths. In the meantime, SARS-CoV-2 has become endemic and is now part of the repertoire of viruses causing seasonal severe respiratory infections. Due to several factors, among them the development of SARS-CoV-2 immunity through natural infection, vaccination and the current dominance of seemingly less pathogenic strains belonging to the omicron lineage, the COVID-19 situation has stabilized. However, several challenges remain and the possible new occurrence of highly pathogenic variants remains a threat. Here we review the development, features and importance of assays measuring SARS-CoV-2 neutralizing antibodies (NAbs). In particular we focus on in vitro infection assays and molecular interaction assays studying the binding of the receptor binding domain (RBD) with its cognate cellular receptor ACE2. These assays, but not the measurement of SARS-CoV-2-specific antibodies per se, can inform us of whether antibodies produced by convalescent or vaccinated subjects may protect against the infection and thus have the potential to predict the risk of becoming newly infected. This information is extremely important given the fact that a considerable number of subjects, in particular vulnerable persons, respond poorly to the vaccination with the production of neutralizing antibodies. Furthermore, these assays allow to determine and evaluate the virus-neutralizing capacity of antibodies induced by vaccines and administration of plasma-, immunoglobulin preparations, monoclonal antibodies, ACE2 variants or synthetic compounds to be used for therapy of COVID-19 and assist in the preclinical evaluation of vaccines. Both types of assays can be relatively quickly adapted to newly emerging virus variants to inform us about the magnitude of cross-neutralization, which may even allow us to estimate the risk of becoming infected by new
Weiterlesen »
 Study suggests saliva samples may be better indicators of SARS-CoV-2 persistence than nasal swabsIn a recent study published in the PLOS ONE journal, researchers at Rutgers New Jersey Medical School explored the persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human saliva.
Study suggests saliva samples may be better indicators of SARS-CoV-2 persistence than nasal swabsIn a recent study published in the PLOS ONE journal, researchers at Rutgers New Jersey Medical School explored the persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human saliva.
Weiterlesen »
 Study provides insights on stability of SARS-CoV-2 in biological fluids of animalsIn a recent study published in the Viruses journal, researchers assessed the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among biological fluids found in animals.
Study provides insights on stability of SARS-CoV-2 in biological fluids of animalsIn a recent study published in the Viruses journal, researchers assessed the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among biological fluids found in animals.
Weiterlesen »
 Post COVID-19 irritable bowel syndromeObjectives The long-term consequences of COVID-19 infection on the gastrointestinal tract remain unclear. Here, we aimed to evaluate the prevalence of gastrointestinal symptoms and post-COVID-19 disorders of gut–brain interaction after hospitalisation for SARS-CoV-2 infection. Design GI-COVID-19 is a prospective, multicentre, controlled study. Patients with and without COVID-19 diagnosis were evaluated on hospital admission and after 1, 6 and 12 months post hospitalisation. Gastrointestinal symptoms, anxiety and depression were assessed using validated questionnaires. Results The study included 2183 hospitalised patients. The primary analysis included a total of 883 patients (614 patients with COVID-19 and 269 controls) due to the exclusion of patients with pre-existing gastrointestinal symptoms and/or surgery. At enrolment, gastrointestinal symptoms were more frequent among patients with COVID-19 than in the control group (59.3% vs 39.7%, p|0.001). At the 12-month follow-up, constipation and hard stools were significantly more prevalent in controls than in patients with COVID-19 (16% vs 9.6%, p=0.019 and 17.7% vs 10.9%, p=0.011, respectively). Compared with controls, patients with COVID-19 reported higher rates of irritable bowel syndrome (IBS) according to Rome IV criteria: 0.5% versus 3.2%, p=0.045. Factors significantly associated with IBS diagnosis included history of allergies, chronic intake of proton pump inhibitors and presence of dyspnoea. At the 6-month follow-up, the rate of patients with COVID-19 fulfilling the criteria for depression was higher than among controls. Conclusion Compared with controls, hospitalised patients with COVID-19 had fewer problems of constipation and hard stools at 12 months after acute infection. Patients with COVID-19 had significantly higher rates of IBS than controls. Trial registration number [NCT04691895][1]. Data are available upon reasonable request. Data are available on reasonable request. All figures have associated ra
Post COVID-19 irritable bowel syndromeObjectives The long-term consequences of COVID-19 infection on the gastrointestinal tract remain unclear. Here, we aimed to evaluate the prevalence of gastrointestinal symptoms and post-COVID-19 disorders of gut–brain interaction after hospitalisation for SARS-CoV-2 infection. Design GI-COVID-19 is a prospective, multicentre, controlled study. Patients with and without COVID-19 diagnosis were evaluated on hospital admission and after 1, 6 and 12 months post hospitalisation. Gastrointestinal symptoms, anxiety and depression were assessed using validated questionnaires. Results The study included 2183 hospitalised patients. The primary analysis included a total of 883 patients (614 patients with COVID-19 and 269 controls) due to the exclusion of patients with pre-existing gastrointestinal symptoms and/or surgery. At enrolment, gastrointestinal symptoms were more frequent among patients with COVID-19 than in the control group (59.3% vs 39.7%, p|0.001). At the 12-month follow-up, constipation and hard stools were significantly more prevalent in controls than in patients with COVID-19 (16% vs 9.6%, p=0.019 and 17.7% vs 10.9%, p=0.011, respectively). Compared with controls, patients with COVID-19 reported higher rates of irritable bowel syndrome (IBS) according to Rome IV criteria: 0.5% versus 3.2%, p=0.045. Factors significantly associated with IBS diagnosis included history of allergies, chronic intake of proton pump inhibitors and presence of dyspnoea. At the 6-month follow-up, the rate of patients with COVID-19 fulfilling the criteria for depression was higher than among controls. Conclusion Compared with controls, hospitalised patients with COVID-19 had fewer problems of constipation and hard stools at 12 months after acute infection. Patients with COVID-19 had significantly higher rates of IBS than controls. Trial registration number [NCT04691895][1]. Data are available upon reasonable request. Data are available on reasonable request. All figures have associated ra
Weiterlesen »
