Pfizer is recalling more than four million packages of migraine medication.
Pfizer on March 16, 2023, recalled 4.2 million packages of the prescription drug Nurtec ODT, used to treat migraines. The medication was recalled because the packaging is not child-resistant and could be poisonous to children, according to the U.S. Consumer Product Safety Commission.
The company is recalling around 4.2 million units of Nurtec ODT 75mg orally disintegrating tablets, which are sold in packs of eight doses on a blister card, on Thursday, the U.S. Consumer Product Safety Commission said. “The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children,” it warned.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
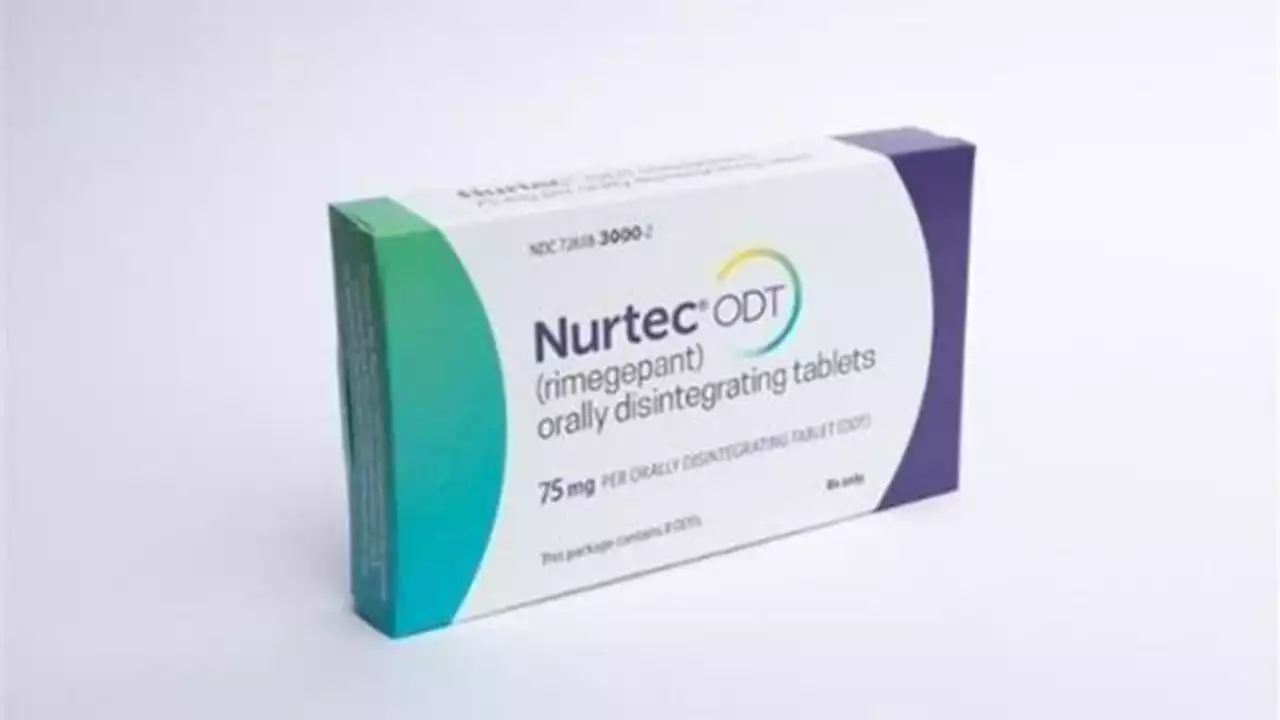 Nurtec ODT recall: Pfizer to develop new packaging after product didn't meet child-resistant requirementsPfizer is recalling 4.2 million packages of Nurtec ODT 75 mg orally disintegrating tablets for failing to meet child-resistant requirements, according to safety regulators.
Nurtec ODT recall: Pfizer to develop new packaging after product didn't meet child-resistant requirementsPfizer is recalling 4.2 million packages of Nurtec ODT 75 mg orally disintegrating tablets for failing to meet child-resistant requirements, according to safety regulators.
Weiterlesen »
 U.S. FDA advisers vote in favor of full approval for Pfizer's COVID pill PaxlovidAdvisers to the U.S. health regulator on Thursday voted in favor of recommending a full approval for Pfizer's COVID-19 pill Paxlovid in adults at high risk of progression to severe disease.
U.S. FDA advisers vote in favor of full approval for Pfizer's COVID pill PaxlovidAdvisers to the U.S. health regulator on Thursday voted in favor of recommending a full approval for Pfizer's COVID-19 pill Paxlovid in adults at high risk of progression to severe disease.
Weiterlesen »
 FDA advisers back Pfizer's COVID treatment for full approvalAdvisers to the U.S. Food and Drug Administration on Thursday overwhelmingly backed full approval of Pfizer's oral antiviral COVID-19 treatment Paxlovid for adults at high risk of progression to severe disease.
FDA advisers back Pfizer's COVID treatment for full approvalAdvisers to the U.S. Food and Drug Administration on Thursday overwhelmingly backed full approval of Pfizer's oral antiviral COVID-19 treatment Paxlovid for adults at high risk of progression to severe disease.
Weiterlesen »
 FDA advisers vote in favor of full approval for Pfizer's COVID pill PaxlovidU.S. health regulators voted in favor of a full approval for Pfizer's COVID pill Paxlovid. This pill will will help adults who are at high risk of progression to severe virus.
FDA advisers vote in favor of full approval for Pfizer's COVID pill PaxlovidU.S. health regulators voted in favor of a full approval for Pfizer's COVID pill Paxlovid. This pill will will help adults who are at high risk of progression to severe virus.
Weiterlesen »
 Pfizer ‘recalls’ 4.2 million boxes of Nurtec migraine medicine due to lack of childproofingPharmaceutical company Pfizer is “recalling” 4.2 million units of its migraine medication Nurtec because the single eight-count blister pack in each box is not adequately child-proofed.
Pfizer ‘recalls’ 4.2 million boxes of Nurtec migraine medicine due to lack of childproofingPharmaceutical company Pfizer is “recalling” 4.2 million units of its migraine medication Nurtec because the single eight-count blister pack in each box is not adequately child-proofed.
Weiterlesen »
