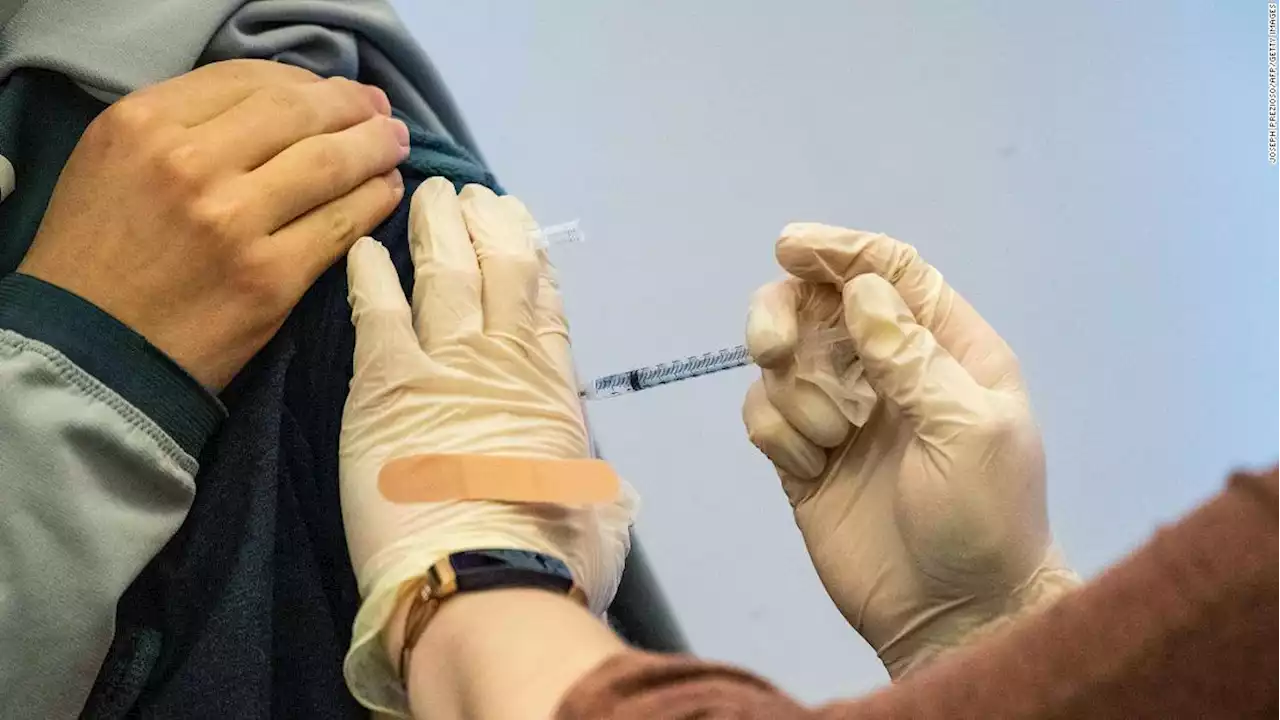
Pfizer and BioNTech submitted an application for emergency use authorization for a second booster dose of their Covid-19 vaccine for adults 65 and older
The submission is based on two data sets from Israel."Both data sets showed evidence that an additional mRNA booster increases immunogenicity and lowers rates of confirmed infections and severe illness," the companies said in a news release.Here's what could lie ahead for the US in the third year of the pandemic Pfizer CEO Albert Bourla said Sunday that he expects that a fourth dose of Covid-19 vaccine will be needed."It is necessary -- a fourth boost for right now.
Read MoreSome countries are offering fourth vaccine doses, especially to people at higher risk of severe disease and death. In the United States, certain immunocompromised adults can receive three doses of the coronavirus vaccine and a fourth shot as a booster dose. Two doses of the Pfizer/BioNTech coronavirus vaccine are available for people 5 and older, and people 12 and older are eligible to receive a booster five months after their second shot.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 Pfizer and BioNTech plan to seek authorization for second COVID booster for people 65 and upNEW: Pfizer and its partner BioNTech plan to seek emergency authorization for a second COVID booster shot for people 65 and older, Axios has confirmed.
Pfizer and BioNTech plan to seek authorization for second COVID booster for people 65 and upNEW: Pfizer and its partner BioNTech plan to seek emergency authorization for a second COVID booster shot for people 65 and older, Axios has confirmed.
Weiterlesen »
 Pfizer to seek authorization for 2nd Covid booster for people 65 and olderIf the FDA authorizes it, the additional shot would go to people who are among those with the highest risk of serious illness.
Pfizer to seek authorization for 2nd Covid booster for people 65 and olderIf the FDA authorizes it, the additional shot would go to people who are among those with the highest risk of serious illness.
Weiterlesen »
 Covid-19: One Covid-related death and 463 in hospitalThe total number of deaths linked to the virus reported by Stormont's Department of Health is 3,253.
Covid-19: One Covid-related death and 463 in hospitalThe total number of deaths linked to the virus reported by Stormont's Department of Health is 3,253.
Weiterlesen »
 Covid-19: Five Covid-related deaths and 506 in hospitalThe total number of deaths linked to the virus reported by Stormont's Department of Health is 3,258.
Covid-19: Five Covid-related deaths and 506 in hospitalThe total number of deaths linked to the virus reported by Stormont's Department of Health is 3,258.
Weiterlesen »
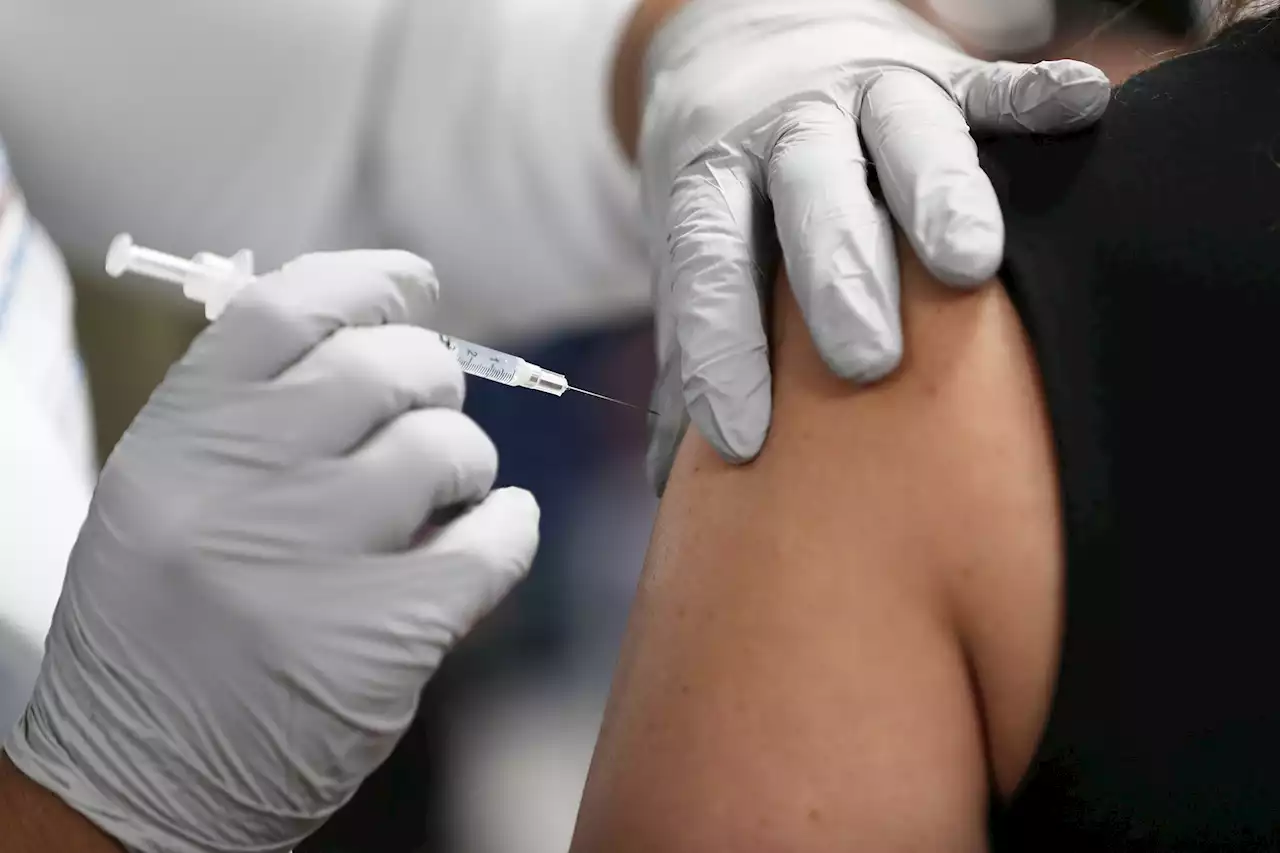 Pfizer CEO says fourth COVID-19 shot ‘necessary’ due to waning immunityMost people will need to get fourth COVID-19 vaccine dose to be protected from the virus, Pfizer CEO Albert Bourla said on “Face The Nation” on Sunday.
Pfizer CEO says fourth COVID-19 shot ‘necessary’ due to waning immunityMost people will need to get fourth COVID-19 vaccine dose to be protected from the virus, Pfizer CEO Albert Bourla said on “Face The Nation” on Sunday.
Weiterlesen »
 Pfizer: 4th COVID-19 vaccine dose will be needed“Right now, the way that we have seen, it is necessary, a fourth booster right now,” Pfizer’s CEO Albert Bourla said. FOX13
Pfizer: 4th COVID-19 vaccine dose will be needed“Right now, the way that we have seen, it is necessary, a fourth booster right now,” Pfizer’s CEO Albert Bourla said. FOX13
Weiterlesen »