Paxlovid not linked to COVID rebound, FDA says.
- Advisers for the Food and Drug Administration are meeting Thursday to consider full approval for Pfizer’s Paxlovid.
The FDA gave the COVID-19 anti-viral emergency use authorization in 2021 for treating mild to moderate illness in high-risk adults.Some users had reported a return of symptoms and a positive COVID-19 test after finishing the five-day course of Paxlovid. However, clinical trial data has concluded that there is no clear association with a COVID-19 rebound.The FDA is expected to complete its review for approval in May.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
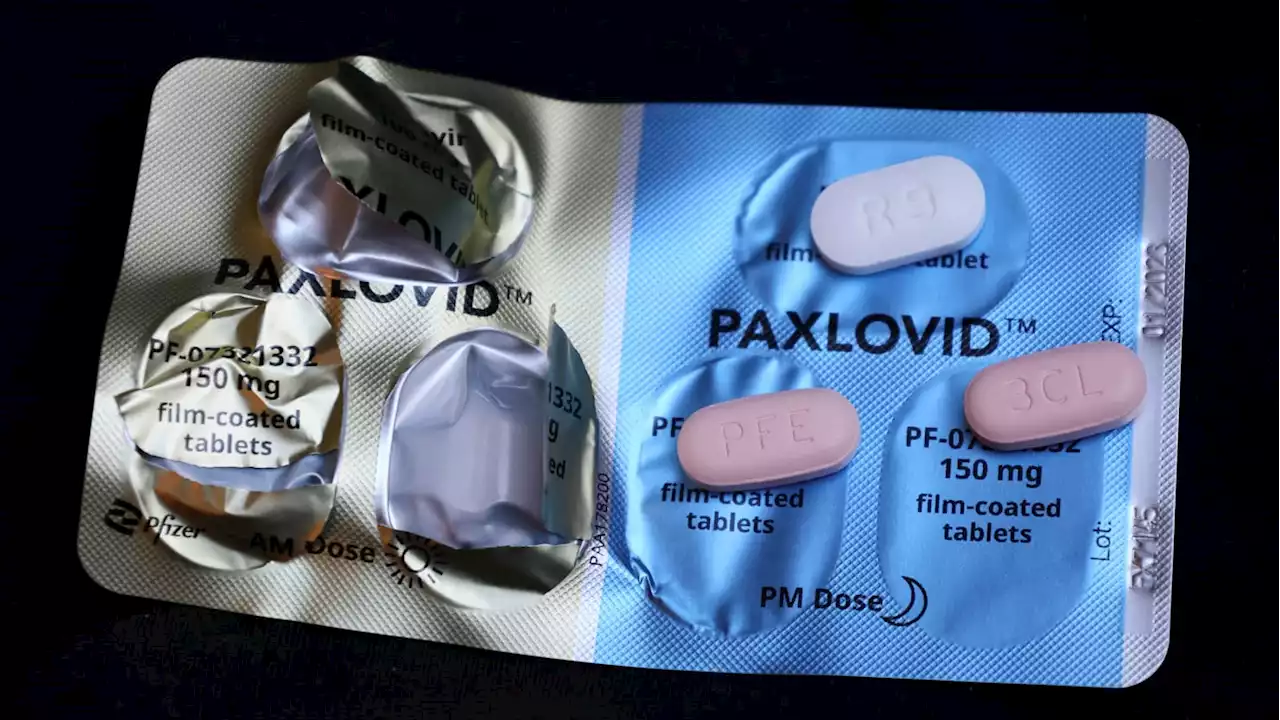 FDA: Paxlovid Does Not Cause COVID ‘Rebound’The FDA says in a new report that rebound also occurs in people who did not take paxlovid—which can prevent COVID from becoming severe if given early enough—and appears to be part of the disease process in some.
FDA: Paxlovid Does Not Cause COVID ‘Rebound’The FDA says in a new report that rebound also occurs in people who did not take paxlovid—which can prevent COVID from becoming severe if given early enough—and appears to be part of the disease process in some.
Weiterlesen »
 FDA: Paxlovid not associated with COVID reboundPaxlovid isn't associated with COVID rebound, in which patients test positive or have symptoms days after a course of the drug is completed, Food and Drug Administration staff said Tuesday.
FDA: Paxlovid not associated with COVID reboundPaxlovid isn't associated with COVID rebound, in which patients test positive or have symptoms days after a course of the drug is completed, Food and Drug Administration staff said Tuesday.
Weiterlesen »
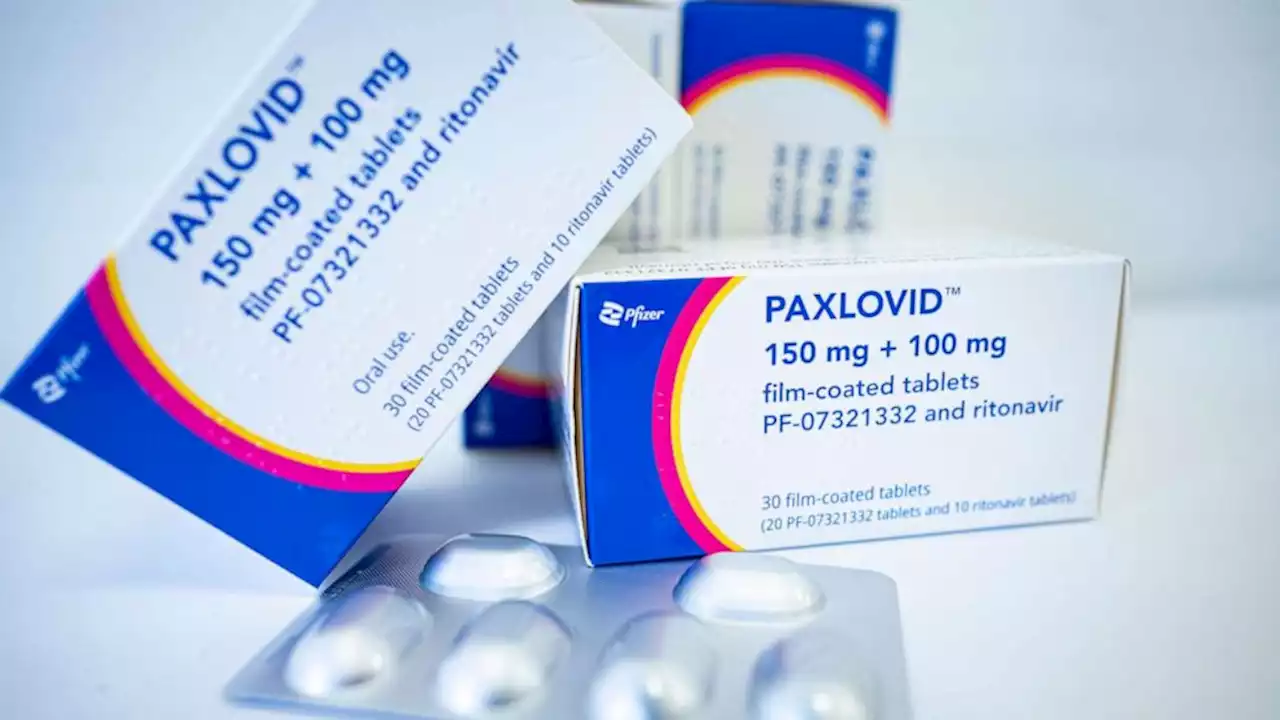 No clear association between Paxlovid and COVID-19 rebound, FDA saysAn FDA analysis did not find a clear association between the COVID-19 antiviral drug Paxlovid and illness rebound, the FDA said in a new report.
No clear association between Paxlovid and COVID-19 rebound, FDA saysAn FDA analysis did not find a clear association between the COVID-19 antiviral drug Paxlovid and illness rebound, the FDA said in a new report.
Weiterlesen »
 FDA panel to meet Thursday to discuss full approval of PaxlovidA Food and Drug Administration committee is set to meet Thursday to decide whether the regulator should grant full approval to Paxlovid, the antiviral pill...
FDA panel to meet Thursday to discuss full approval of PaxlovidA Food and Drug Administration committee is set to meet Thursday to decide whether the regulator should grant full approval to Paxlovid, the antiviral pill...
Weiterlesen »
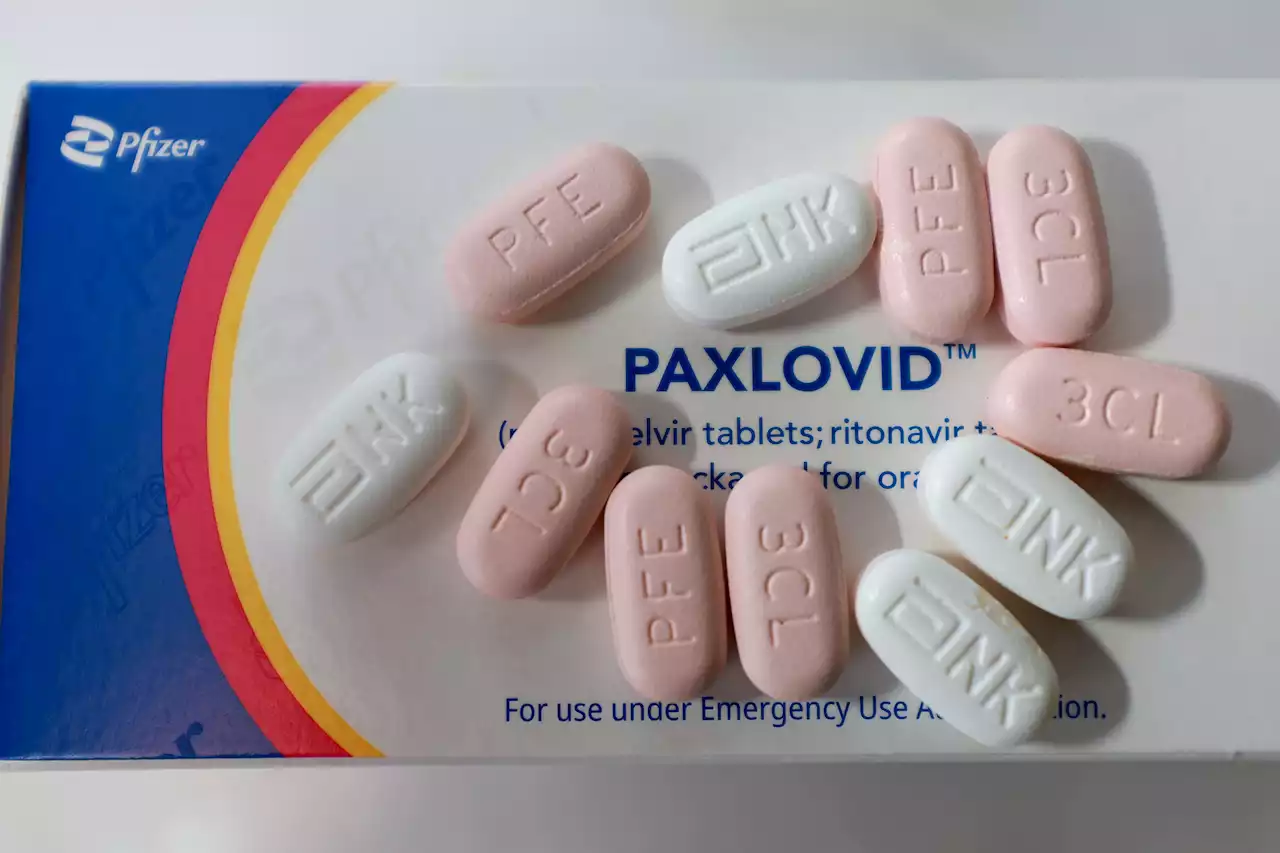 FDA Staff Report Says Data Shows Pfizer's Covid Treatment Paxlovid Is Effective in High-Risk AdultsFDA staff said Pfizer’s clinical trial results on its Covid antiviral pill Paxlovid support its use in adults at high risk of progressing to severe disease.
FDA Staff Report Says Data Shows Pfizer's Covid Treatment Paxlovid Is Effective in High-Risk AdultsFDA staff said Pfizer’s clinical trial results on its Covid antiviral pill Paxlovid support its use in adults at high risk of progressing to severe disease.
Weiterlesen »
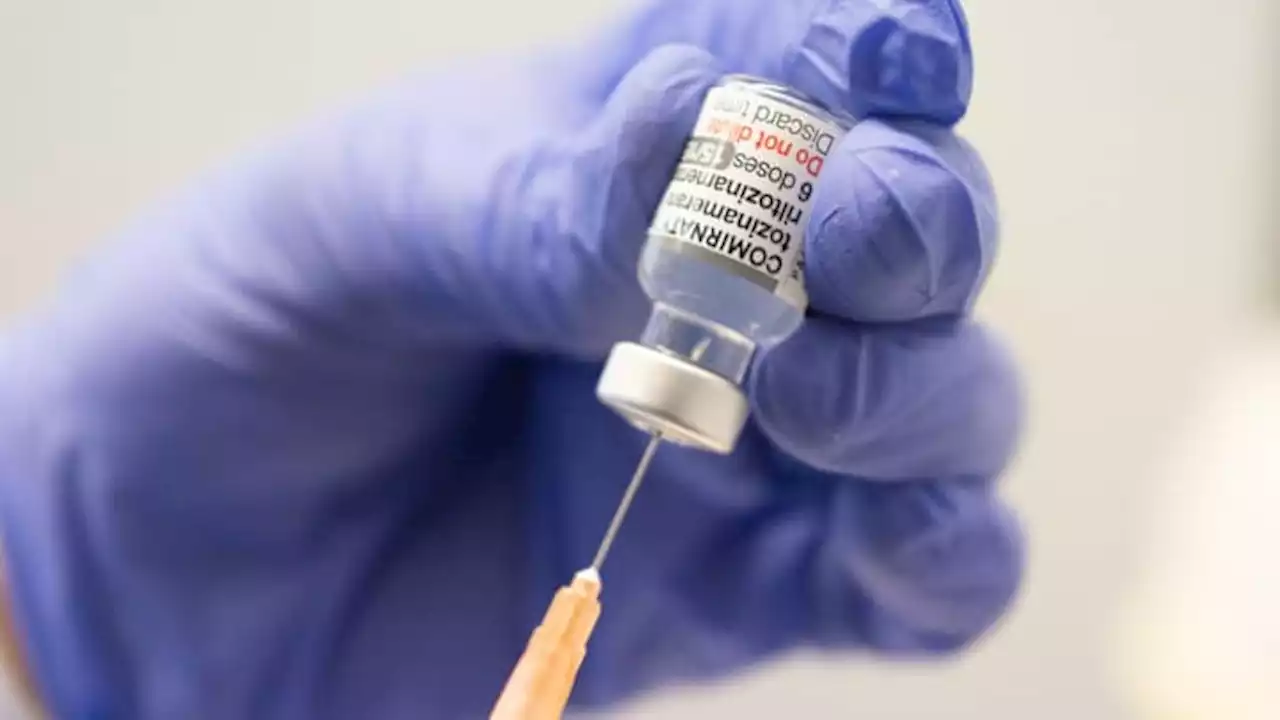 FDA authorizes Pfizer's Covid omicron booster as fourth shot for kids under 5The FDA authorized Pfizer's omicron booster shot for kids younger than five who were previously vaccinated with three doses of the company's original shot.
FDA authorizes Pfizer's Covid omicron booster as fourth shot for kids under 5The FDA authorized Pfizer's omicron booster shot for kids younger than five who were previously vaccinated with three doses of the company's original shot.
Weiterlesen »
