New vaccine offered to fight COVID should soon be ready for Americans.
Right now, the Novavax shot is only authorized as an initial immunization series, so those who have gotten one of the three other COVIDreported. The company plans to apply for booster authorization soon.
Novavax hopes its shot will be seen an as an alternative to the most widely used shots from Pfizer and Moderna, which use messenger RNA technology. The third shot option in the United States is a vaccine from Johnson & Johnson. "Today's FDA emergency use authorization of our COVID-19 vaccine provides the U.S. with access to the first protein-based COVID-19 vaccine," Novavax President and CEO Stanley Erck said in a
. "This authorization reflects the strength of our COVID-19 vaccine's efficacy and safety data, and it underscores the critical need to offer another vaccine option for the U.S. population while the pandemic continues."
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 What to Know About Novavax, the Fourth COVID Vaccine Approved by the FDANovavax, a new covid vaccine, was recommended by an FDA panel for emergency use. What you need to know about approval, release date, and efficacy.
What to Know About Novavax, the Fourth COVID Vaccine Approved by the FDANovavax, a new covid vaccine, was recommended by an FDA panel for emergency use. What you need to know about approval, release date, and efficacy.
Weiterlesen »
 FDA authorizes Novavax COVID-19 vaccineThe FDA on Wednesday issued an emergency use authorization for Novavax's COVID-19 vaccine for adults 18 and older.
FDA authorizes Novavax COVID-19 vaccineThe FDA on Wednesday issued an emergency use authorization for Novavax's COVID-19 vaccine for adults 18 and older.
Weiterlesen »
 FDA Clears New COVID-19 Vaccine Option From Novavax for AdultsNearly a quarter of American adults still haven't gotten their primary vaccinations even this late in the COVID pandemic, and experts expect at least some of them to roll up their sleeves for a more conventional option — a protein-based vaccine.
FDA Clears New COVID-19 Vaccine Option From Novavax for AdultsNearly a quarter of American adults still haven't gotten their primary vaccinations even this late in the COVID pandemic, and experts expect at least some of them to roll up their sleeves for a more conventional option — a protein-based vaccine.
Weiterlesen »
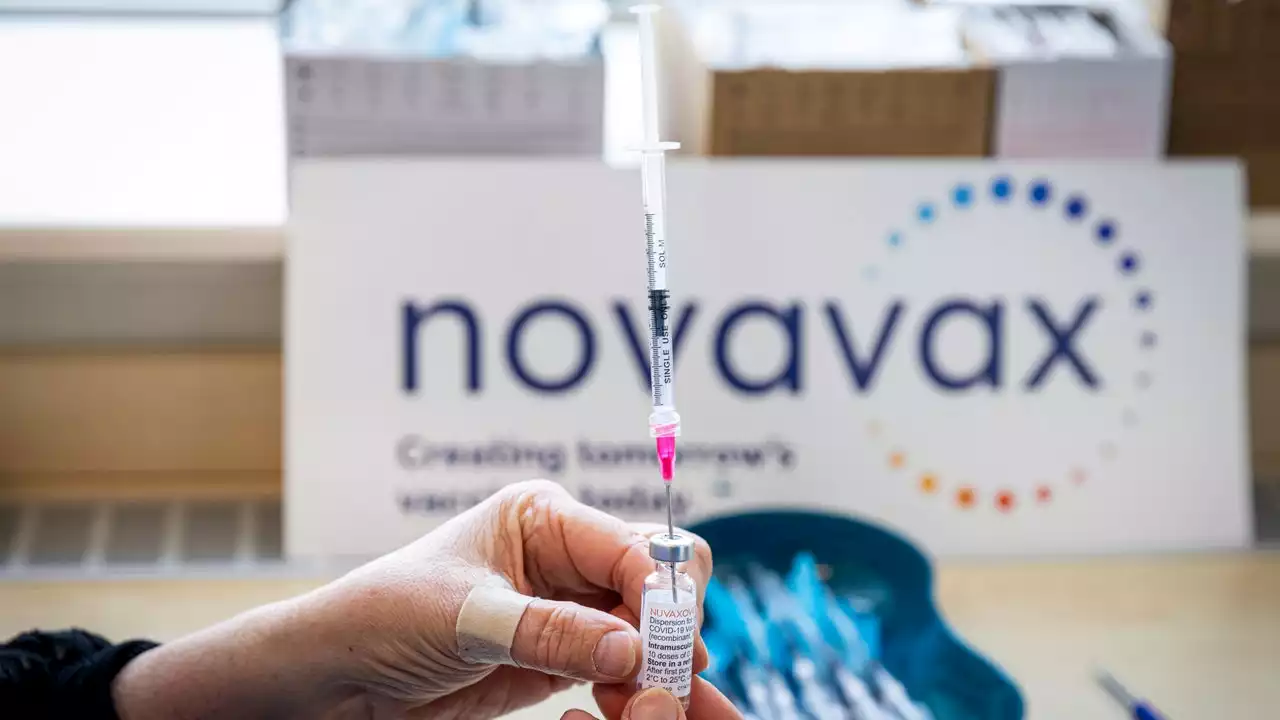 Novavax COVID-19 vaccine: FDA authorizes fourth shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Novavax COVID-19 vaccine: FDA authorizes fourth shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Weiterlesen »
 'Classic type of vaccine': FDA approves new COVID-19 shot from Novavax'It is more the classic type of vaccine that we are used to with say, the influenza vaccine,' said Darren Mareiniss, an emergency medicine physician.
'Classic type of vaccine': FDA approves new COVID-19 shot from Novavax'It is more the classic type of vaccine that we are used to with say, the influenza vaccine,' said Darren Mareiniss, an emergency medicine physician.
Weiterlesen »
 Novavax COVID-19 vaccine: FDA authorizes new shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Novavax COVID-19 vaccine: FDA authorizes new shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Weiterlesen »
