BREAKING: Moderna said it is seeking emergency use authorization for its COVID-19 vaccine for children as young as 6 months old.
Moderna announced Thursday it is seeking emergency use authorization for its COVID-19 vaccine for children as young as 6 months old, which could allow children younger than age 5 to begin getting vaccinated by the summer.
Moderna is seeking authorization for this particular vaccine to be allowed for ages 6 months up to 6 years. Earlier this week, the Centers for Disease Control and Prevention said roughly 75% of children had COVID-19 antibodies by February 2022.“We are proud to share that we have submitted for authorization for our COVID-19 vaccine for young children,” said Stéphane Bancel, CEO of Moderna. “We believe mRNA-1273 will be able to safely protect these children against SARS-CoV-2, which is so important in our continued fight against COVID-19, and will be especially welcomed by parents and caregivers.
Emergency use authorization is given by the Food and Drug Administration for unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases. The Moderna COVID-19 vaccine garnered full FDA approval in January for those ages 18 and over, more than a year after getting emergency use authorization.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Weiterlesen »
 Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Weiterlesen »
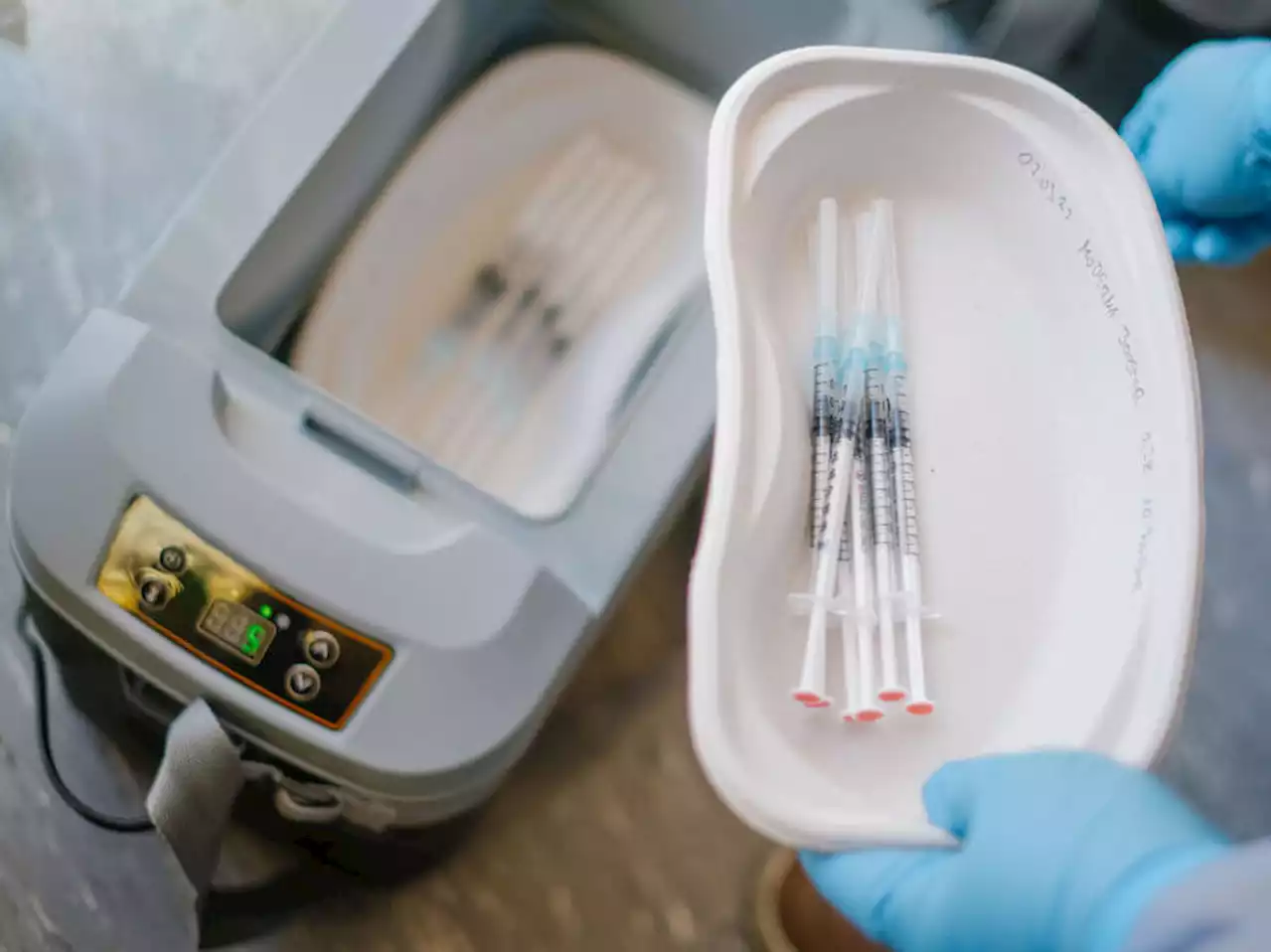 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Weiterlesen »
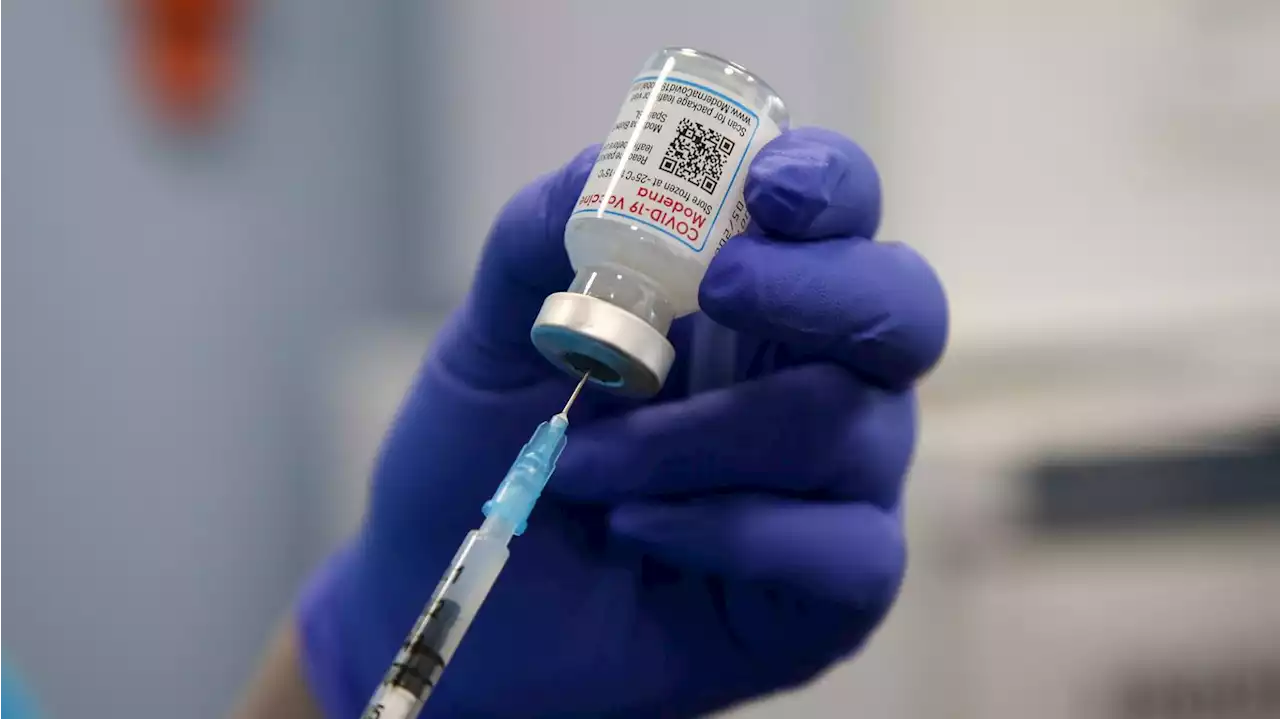 Moderna seeks emergency authorization for COVID-19 vaccine in young childrenNEW: Moderna has submitted a request to the FDA for an emergency use authorization for its COVID-19 vaccine in children 6 months to under 6 years of age.
Moderna seeks emergency authorization for COVID-19 vaccine in young childrenNEW: Moderna has submitted a request to the FDA for an emergency use authorization for its COVID-19 vaccine in children 6 months to under 6 years of age.
Weiterlesen »
 Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Weiterlesen »
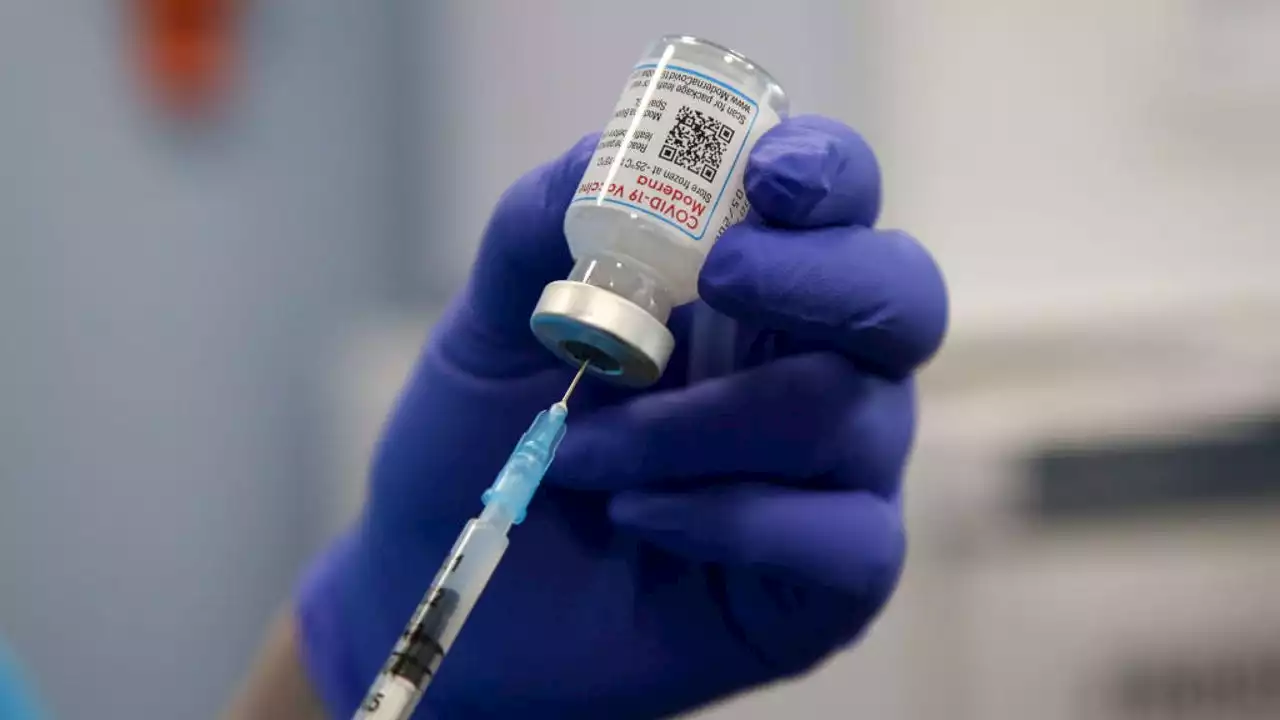 Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Weiterlesen »
