The U.S. Food and Drug Administration (FDA) is not expected to move quickly on regulating cannabinoids (CBD) from hemp, Cowen analyst Harrison Vivas said.
The U.S. Food and Drug Administration is not expected to move quickly on regulating cannabinoids from hemp, Cowen analyst Harrison Vivas said Wednesday in the wake of a special meeting.
“We are encouraged by increasing numbers of studies and greater regulatory focus relative to even five years ago, but we still do not expect to see a near-term change in the FDA’s treatment of CBD in ingestible formats without pressure from Congress,” Vivas said. The FDA cited various animal studies that show negative side effects from consistent consumption at higher levels, but signaled it could consider data from animals with more human-like metabolism of CBD.At Tuesdays FDA meeting, Industry participants laid out a growing body of evidence from third parties that show that CBD can be ingested without dangerous increases in liver enzymes, Vivas noted.
Meanwhile, the FDA continues to educate consumers about potential hazards of CBD as it conducts more research.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 SnoreStop NasoSpray recalled due to microbial contamination, FDA saysGreen Pharmaceuticals, Inc. is notifying retailers and customers to arrange replacements for the recalled product.
SnoreStop NasoSpray recalled due to microbial contamination, FDA saysGreen Pharmaceuticals, Inc. is notifying retailers and customers to arrange replacements for the recalled product.
Weiterlesen »
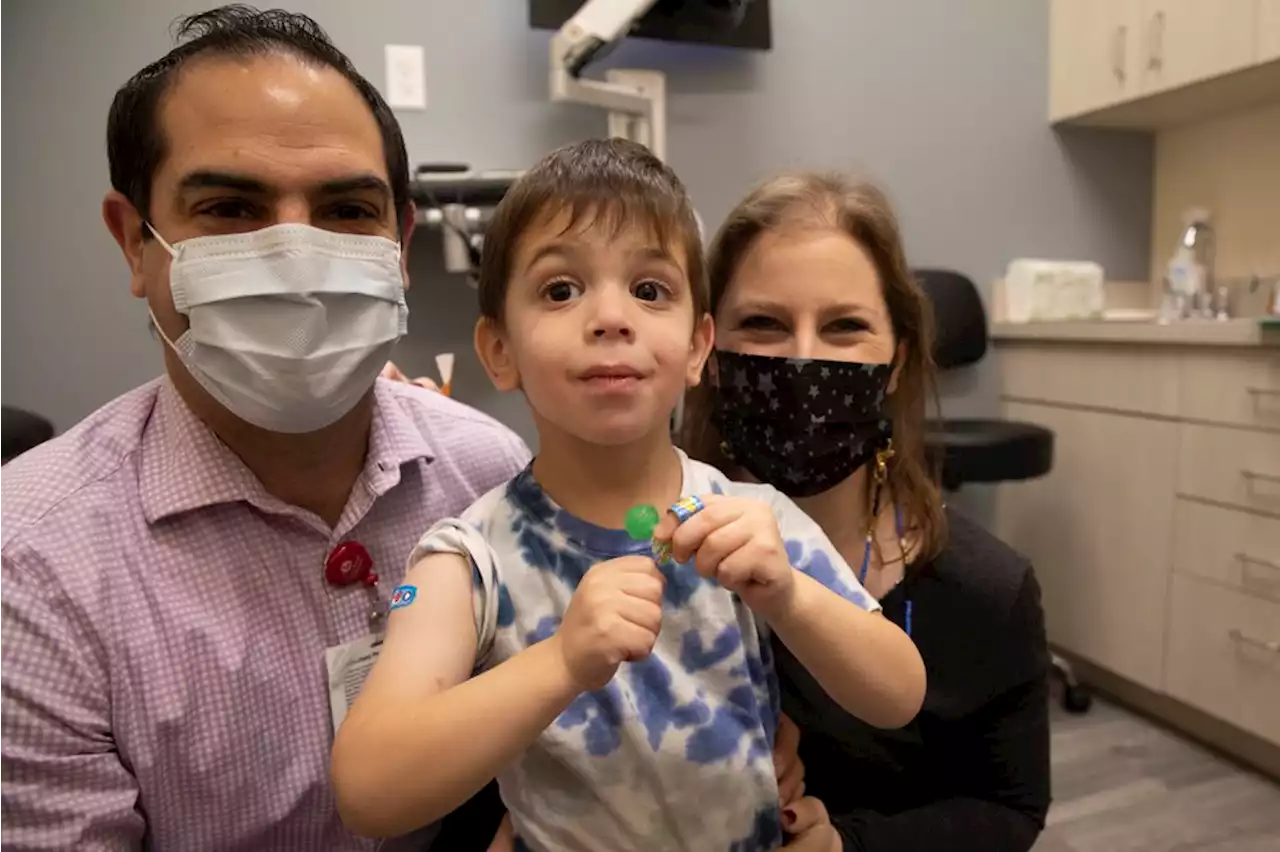 FDA advisers consider Moderna’s COVID shots for teens, younger kidsFDA scientists said there were no confirmed cases of the heart inflammation in Moderna’s kid studies.
FDA advisers consider Moderna’s COVID shots for teens, younger kidsFDA scientists said there were no confirmed cases of the heart inflammation in Moderna’s kid studies.
Weiterlesen »
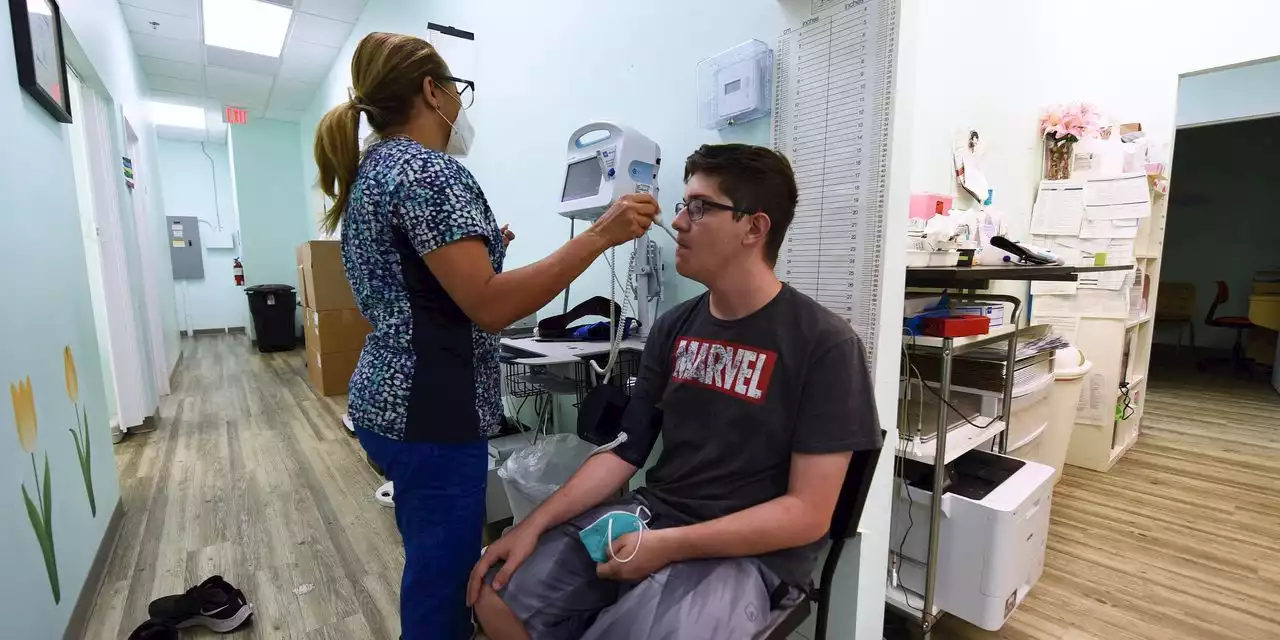 FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
Weiterlesen »
 The FDA Just Approved The First Oral Tablet For Treating Severe Alopecia AreataThe Food and Drug Administration on Monday approved a drug called baricitinib as the first oral tablet for treating severe alopecia areata, an autoimmune disorder affecting more than 300,000 people in the United States every year.
The FDA Just Approved The First Oral Tablet For Treating Severe Alopecia AreataThe Food and Drug Administration on Monday approved a drug called baricitinib as the first oral tablet for treating severe alopecia areata, an autoimmune disorder affecting more than 300,000 people in the United States every year.
Weiterlesen »
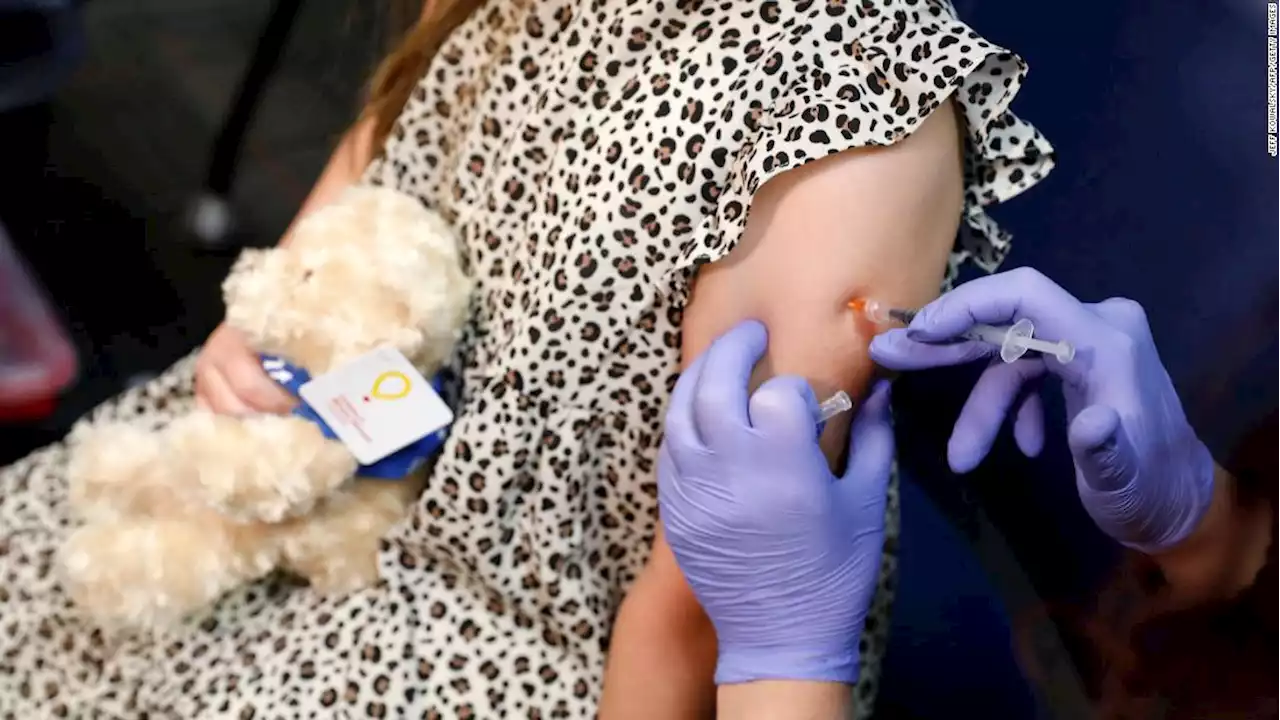 FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
Weiterlesen »
 SnoreStop NasoSpray recalled due to microbial contamination, FDA saysGreen Pharmaceuticals, Inc. is notifying retailers and customers to arrange replacements for the recalled product.
SnoreStop NasoSpray recalled due to microbial contamination, FDA saysGreen Pharmaceuticals, Inc. is notifying retailers and customers to arrange replacements for the recalled product.
Weiterlesen »
