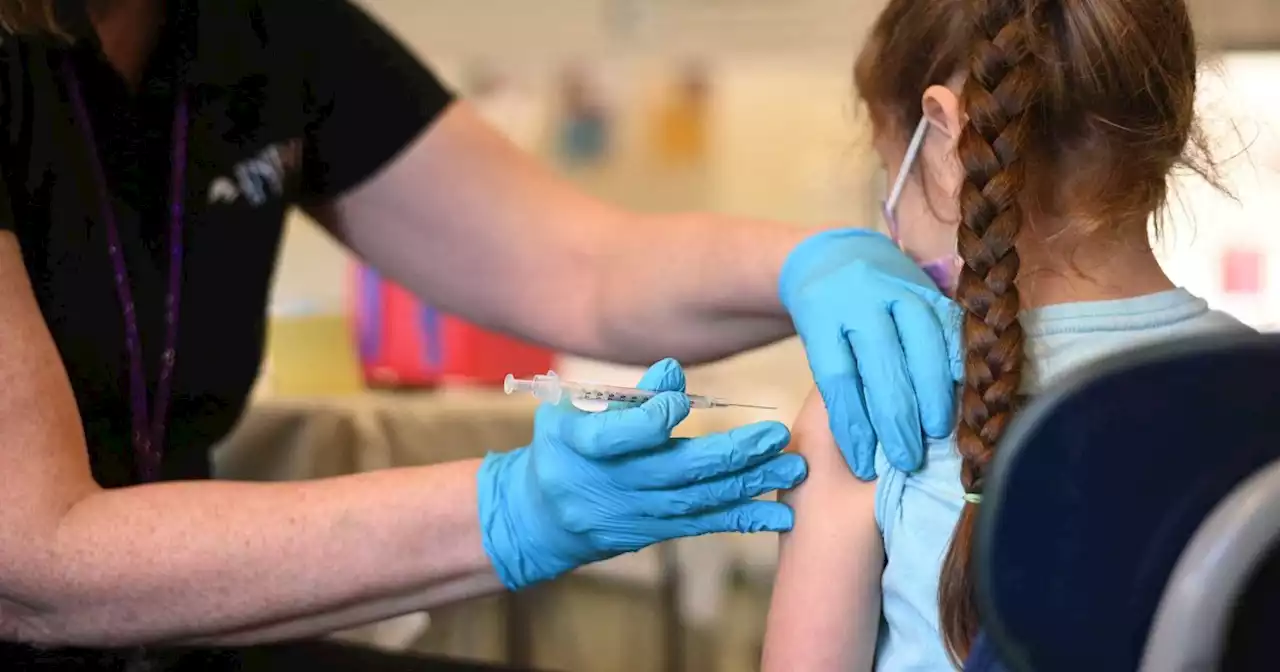
Once the FDA has finalized the dates, it intends to make additional materials available to the public.
Pfizer hasn’t yet submitted an application to the FDA on its three-dose vaccine for kids under 5, but it’s expected to do so in the coming weeks.Novavax’s applicationThe vaccine, which uses proteins to kick-start the body’s immune system, is already available for use in at least 170 countries, but if cleared for emergency use in the U.S., it would provide an alternative to the popular mRNA-based shots from Pfizer and Moderna.
The agency is also planning a June 28 meeting to discuss what virus strain or strains should be included in
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Weiterlesen »
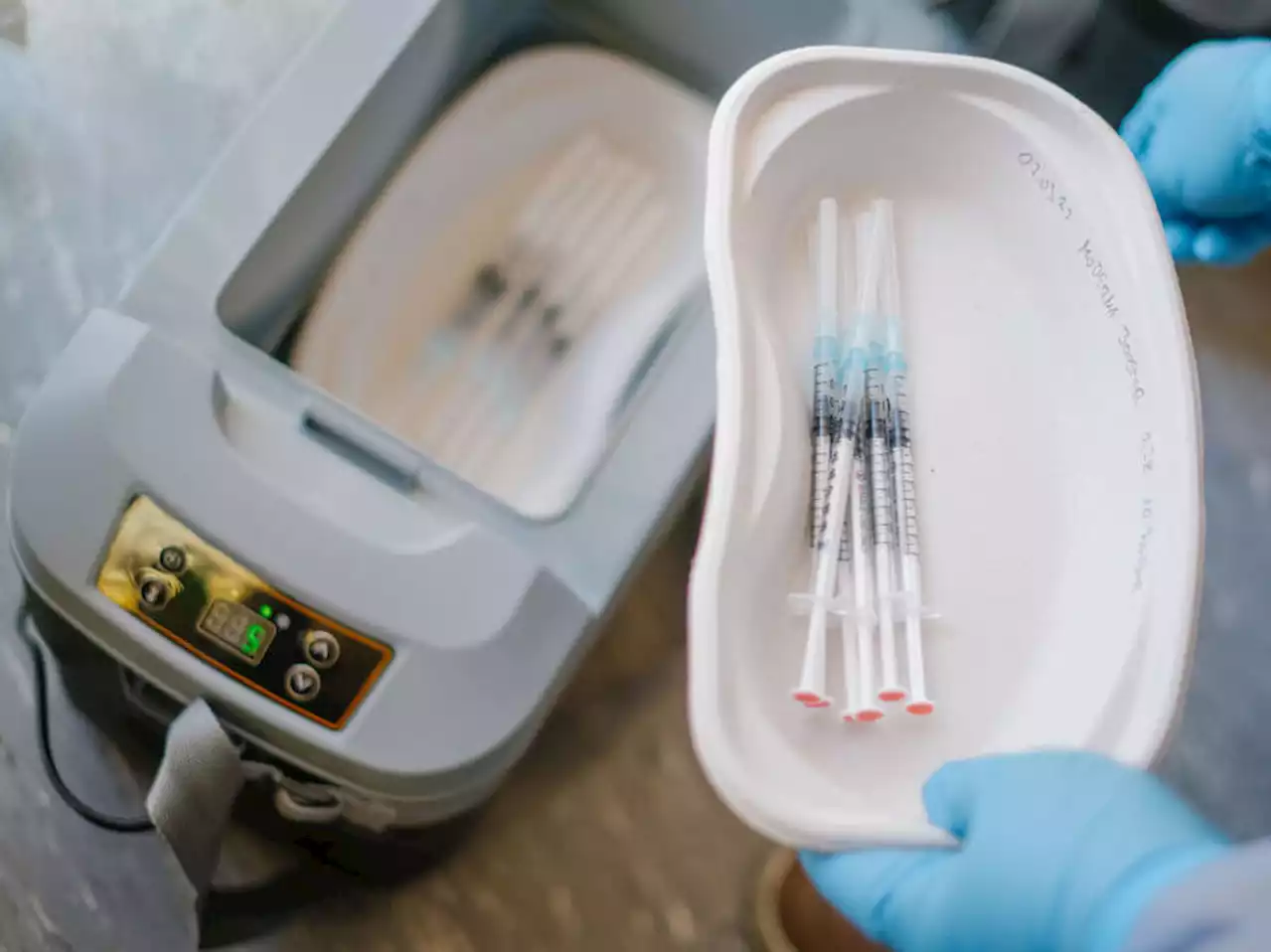 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Weiterlesen »
 Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Weiterlesen »
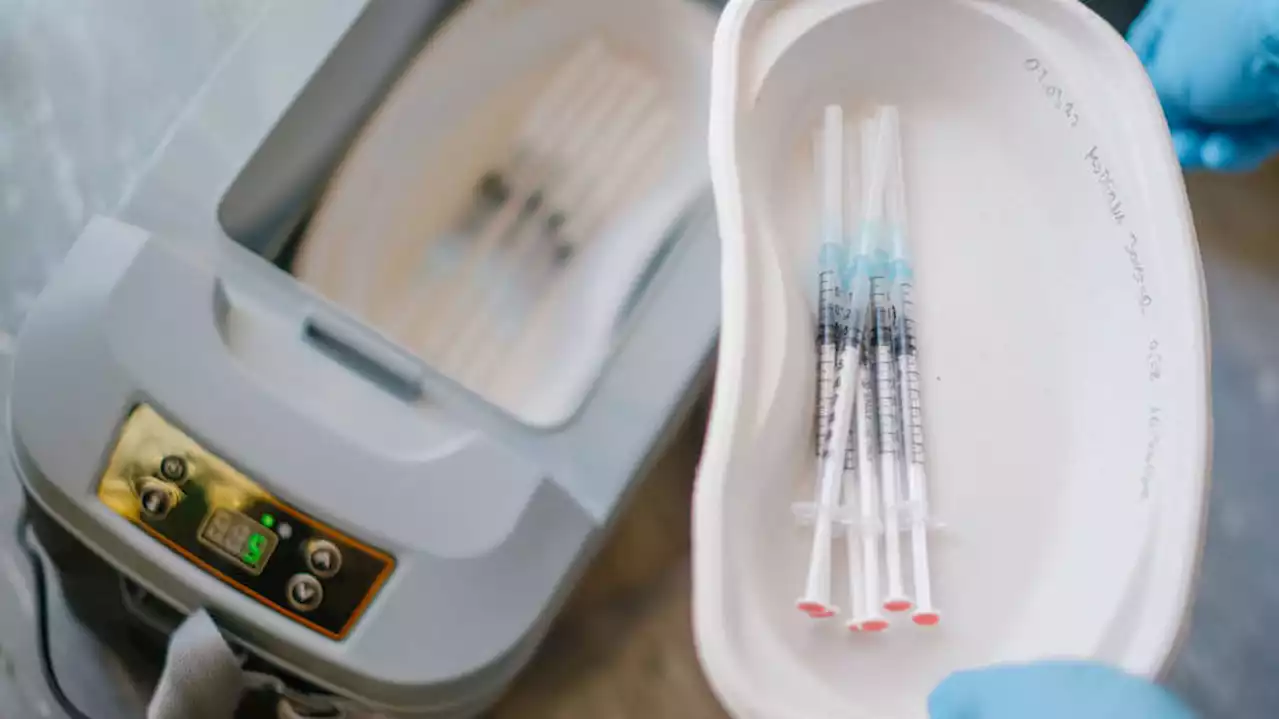 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenModerna announced Thursday that the company has asked the Food and Drug Administration to authorize a low-dose version of its COVID-19 vaccine as the first vaccine for children younger than age 5.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenModerna announced Thursday that the company has asked the Food and Drug Administration to authorize a low-dose version of its COVID-19 vaccine as the first vaccine for children younger than age 5.
Weiterlesen »
 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Weiterlesen »
 Moderna asks FDA to authorize first COVID-19 vaccine for very young children - Alaska Public MediaModerna announced Thursday that the company has asked the Food and Drug Administration to authorize a low-dose version of its COVID-19 vaccine as the first vaccine for children younger than age 5.
Moderna asks FDA to authorize first COVID-19 vaccine for very young children - Alaska Public MediaModerna announced Thursday that the company has asked the Food and Drug Administration to authorize a low-dose version of its COVID-19 vaccine as the first vaccine for children younger than age 5.
Weiterlesen »