A panel of experts voted to approve Pfizer’s new RSV vaccine designed to protect infants from the virus during their most vulnerable first six months of life.
and hospitals pushed to capacity, the first vaccine to designed to reduce severe cases in newborns is now one step closer to approval and rollout.
For the first 90 days after birth, only six infants in the vaccination group had a serious case of RSV, compared with 33 in the placebo group, translating to an efficacy of nearly 82 percent. In the first six months, during which baby is most likely to be hospitalized with a severe case of RSV, only 19 babies in the treatment group fell seriously ill compared to 62 in the placebo group.typically causes mild, cold-like symptoms among most adults and older children.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
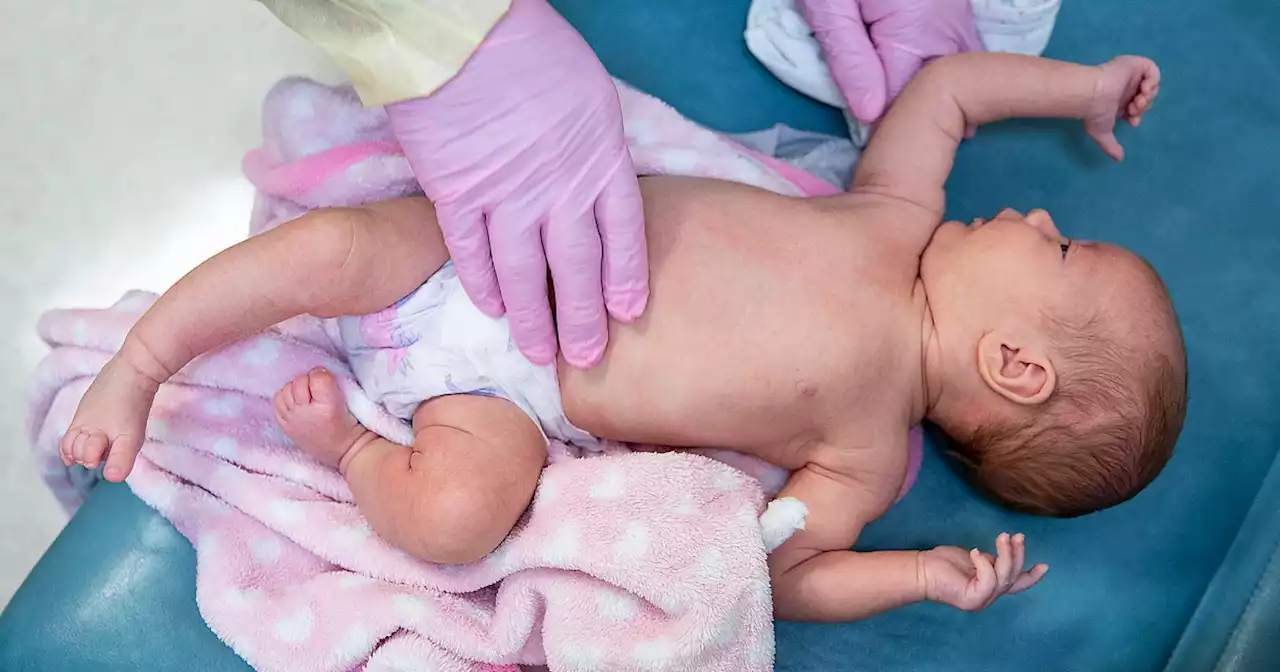 FDA panel to vote on the first RSV vaccine for infants, given to pregnant mothersAn independent advisory committee to the FDA will decide on Thursday whether to recommend an RSV vaccine for infants. If approved, the shot, which is administered to pregnant mothers, would be the first of its kind.
FDA panel to vote on the first RSV vaccine for infants, given to pregnant mothersAn independent advisory committee to the FDA will decide on Thursday whether to recommend an RSV vaccine for infants. If approved, the shot, which is administered to pregnant mothers, would be the first of its kind.
Weiterlesen »
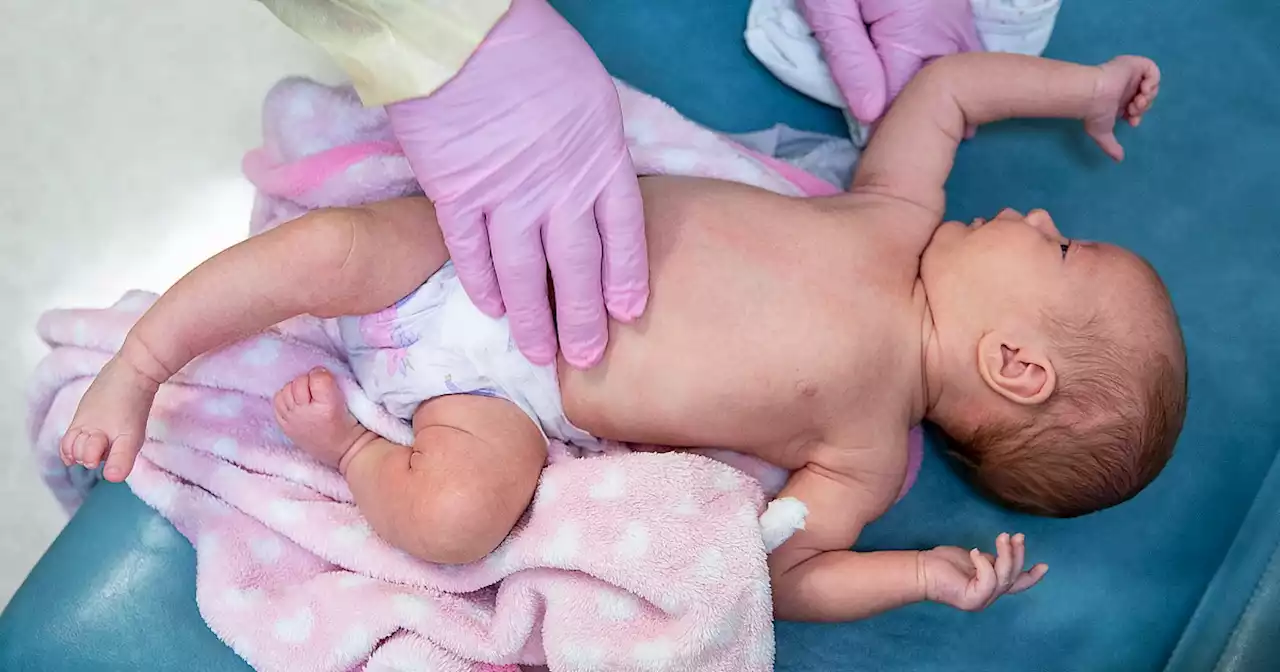 FDA panel to vote on the first RSV vaccine for infants, administered to pregnant mothersIf eventually approved, the shot, which is made by Pfizer and administered to pregnant mothers, would be the first RSV vaccine for infants in the U.S.
FDA panel to vote on the first RSV vaccine for infants, administered to pregnant mothersIf eventually approved, the shot, which is made by Pfizer and administered to pregnant mothers, would be the first RSV vaccine for infants in the U.S.
Weiterlesen »
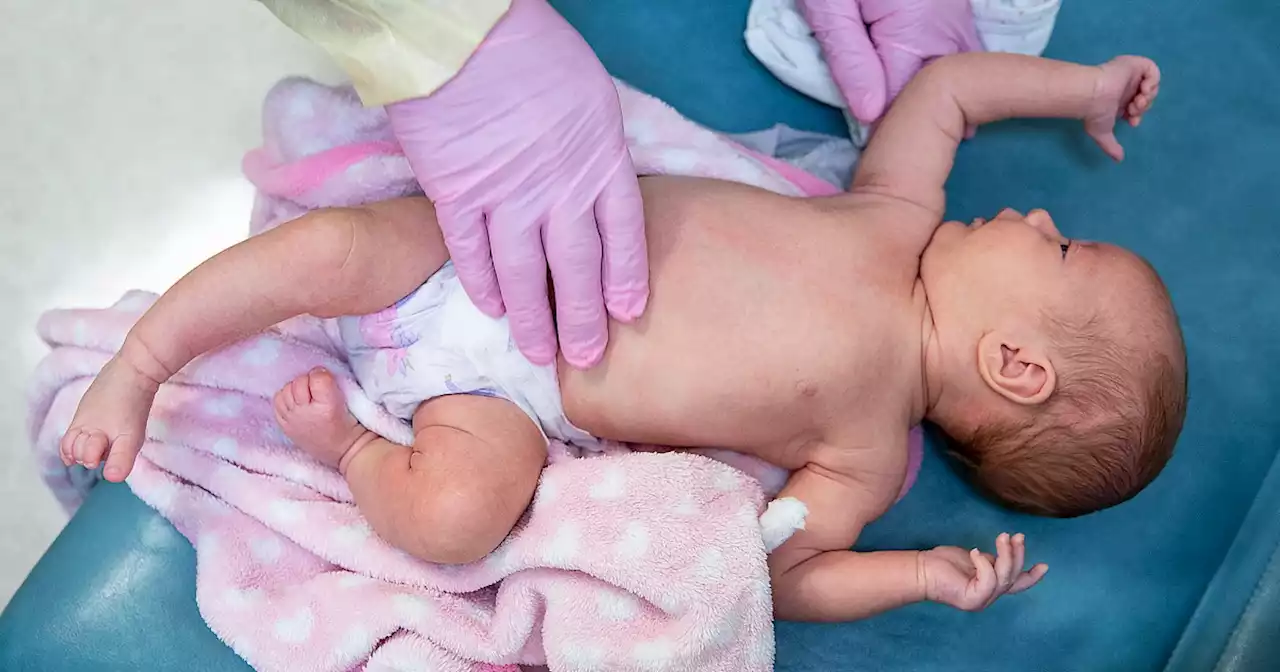 FDA panel recommends the first shot to prevent RSV in infants by vaccinating pregnant mothersBREAKING: FDA panel recommends first shot to prevent RSV in infants by vaccinating pregnant mothers. If the shot gets approved, it would be the first RSV vaccine that confers protection to babies.
FDA panel recommends the first shot to prevent RSV in infants by vaccinating pregnant mothersBREAKING: FDA panel recommends first shot to prevent RSV in infants by vaccinating pregnant mothers. If the shot gets approved, it would be the first RSV vaccine that confers protection to babies.
Weiterlesen »
 First RSV vaccine to protect infants wins backing of FDA panelThe first vaccine to protect babies from the respiratory virus RSV is a step closer to approval, after a panel of the Food and Drug Administration's outside vaccine advisers voted to back the safety and effectiveness of a new shot developed by Pfizer.
First RSV vaccine to protect infants wins backing of FDA panelThe first vaccine to protect babies from the respiratory virus RSV is a step closer to approval, after a panel of the Food and Drug Administration's outside vaccine advisers voted to back the safety and effectiveness of a new shot developed by Pfizer.
Weiterlesen »
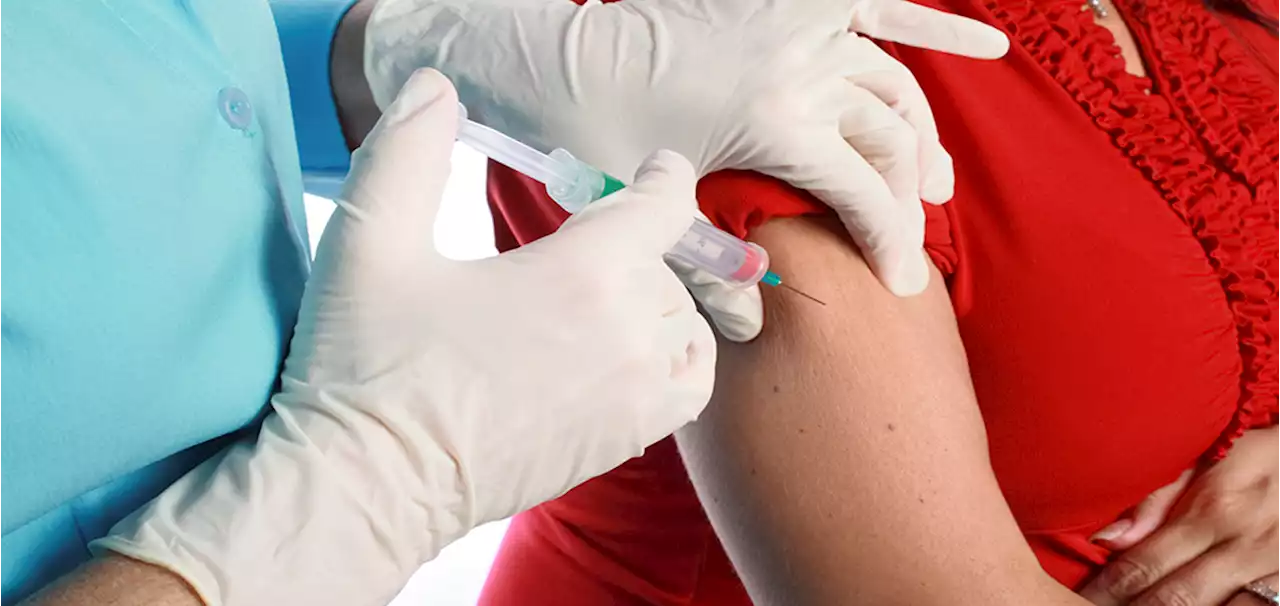 FDA Advisory Panel Recommends RSV Vaccine for Pregnant WomenAn FDA advisory panel has recommended that the FDA approve an RSV vaccine for pregnant women. The vaccine would be given in the final trimester, with protection from RSV being passed through the placenta to the unborn baby.
FDA Advisory Panel Recommends RSV Vaccine for Pregnant WomenAn FDA advisory panel has recommended that the FDA approve an RSV vaccine for pregnant women. The vaccine would be given in the final trimester, with protection from RSV being passed through the placenta to the unborn baby.
Weiterlesen »