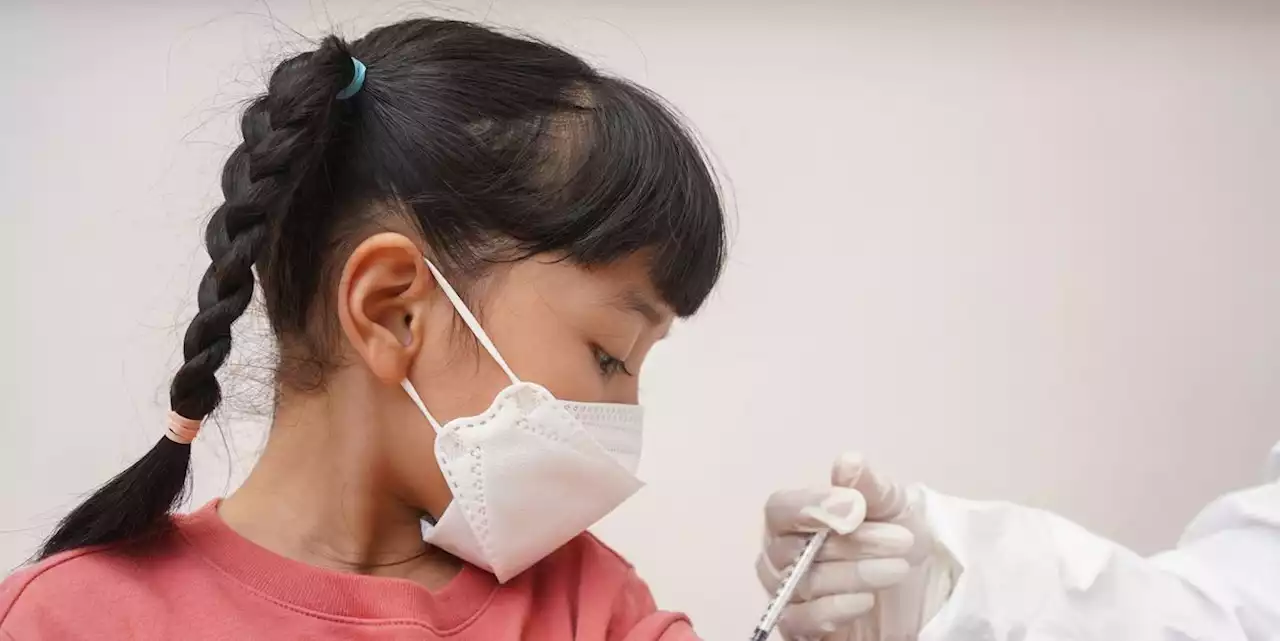
FDA Recommends COVID-19 Vaccines for Children Under 5—What You Need to Know
vaccine is expected to finally become available for children as young as six months as early as next week after an advisory panel to the Food and Drug Administration recommended the shot for this age group. From here, the data for the Pfizer-BioNTech and Moderna vaccines will go to the Centers for Disease Control and Prevention , which is expected to sign off on the vaccine for this age group.of clinical trial data and suggests that children under five—the only age group in the U.S.
Pfizer’s shot will be a three-dose regimen in children, with a 3-microgram dose of the vaccine for each. The vaccine was found to be 80.3% effective at preventing COVID-19 during the Omicron wave, according to “I think we have become numb to the numbers, thinking that they somehow translates into ‘This is a very mild illness that doesn’t affect children’—that’s not true,” Dr. Salazar says. “For any other disease, we would be panicking.”
Amesh A. Adalja, M.D., a senior scholar at the Johns Hopkins Center for Health Security, says that “every child should get this,” noting that it can be scheduled around other pediatric vaccinations if parents prefer. But he’s not hopeful a lot of younger children will get the vaccine. “I don’t expect to see much uptake of this vaccine in the under-five group because there’s not much uptake in the five to 11 group,” he says.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
Weiterlesen »
 FDA Recommends the Emergency Authorization of Moderna's COVID-19 Vaccine for Children Ages 6 to 17An expert panel recommended that kids have another option when it comes to getting vaccinated against COVID-19
FDA Recommends the Emergency Authorization of Moderna's COVID-19 Vaccine for Children Ages 6 to 17An expert panel recommended that kids have another option when it comes to getting vaccinated against COVID-19
Weiterlesen »
 FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens. The expert panel agreed Tuesday that the vaccine made by Moderna is safe and effective enough to give to U.S. kids ages 6 to 17.
FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens. The expert panel agreed Tuesday that the vaccine made by Moderna is safe and effective enough to give to U.S. kids ages 6 to 17.
Weiterlesen »
 FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Weiterlesen »
 FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Weiterlesen »
 FDA panel backs Moderna's COVID-19 vaccine for kids ages 6-17The FDA's outside experts voted unanimously that Moderna’s COVID vaccine is safe and effective enough to give kids ages 6 to 17.
FDA panel backs Moderna's COVID-19 vaccine for kids ages 6-17The FDA's outside experts voted unanimously that Moderna’s COVID vaccine is safe and effective enough to give kids ages 6 to 17.
Weiterlesen »