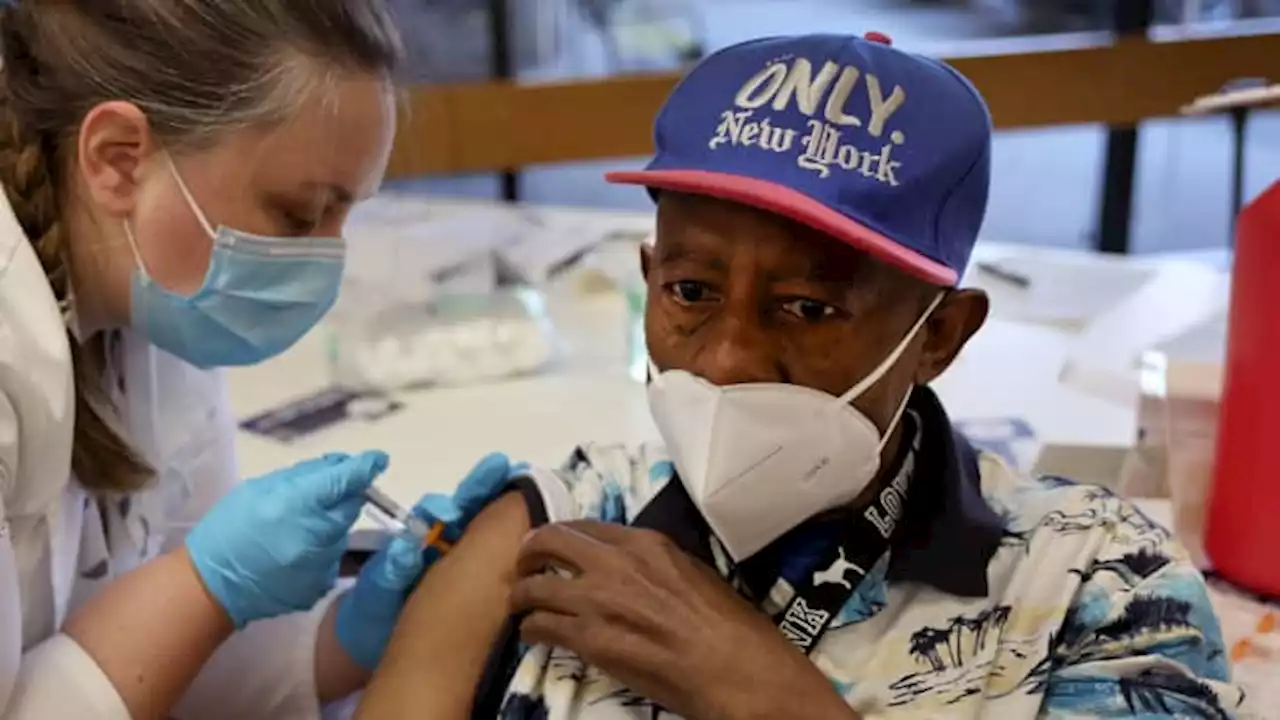
Although the burden of the pandemic has eased substantially, the CDC says Covid continues to kill more than 1,300 people per week.
's Covid-19 vaccines targeting the omicron variant for seniors and people with weak immune systems.
Seniors who are 65 years of age or older and who have already received a vaccine targeting the omicron BA.5 subvariant are now eligible to receive another dose at least four months after their last shot,. People with weak immune systems can receive another omicron shot at least two months after their last dose and receive additional shots at the discretion of their doctor.
Children 6 months through 5 years of age who are unvaccinated can now receive the full two-dose series of Moderna's omicron vaccine. Kids 6 months through 4 years of age can receive three doses of Pfizer's shot that targets omicron.
Although the burden of the pandemic has eased substantially, Covid continues to kill more than 1,300 people per week, according to the Centers for Disease Control and Prevention. Some 1,600 people are still hospitalized with Covid daily on average, according to the public health agency. "COVID-19 continues to be a very real risk for many people, and we encourage individuals to consider staying current with vaccination, including with a bivalent COVID-19 vaccine," said Dr. Peter Marks, who heads the FDA department responsible for vaccines.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA okays second omicron booster for people at high risk from covidBreaking news: FDA clears a second omicron-targeting booster shot for people who are at high risk from the coronavirus
FDA okays second omicron booster for people at high risk from covidBreaking news: FDA clears a second omicron-targeting booster shot for people who are at high risk from the coronavirus
Weiterlesen »
 FDA authorizes second bivalent COVID booster for older adultsThe U.S. Food and Drug Administration on Tuesday authorized a second dose of Omicron-targeting COVID-19 vaccines for older adults as well as those with a weak immune system.
FDA authorizes second bivalent COVID booster for older adultsThe U.S. Food and Drug Administration on Tuesday authorized a second dose of Omicron-targeting COVID-19 vaccines for older adults as well as those with a weak immune system.
Weiterlesen »
 FDA clears second bivalent COVID boosters for some AmericansSome Americans will soon be able to get another shot of the bivalent COVID-19 vaccines, the FDA authorized Tuesday, including seniors ages 65 and older and people with compromised immune systems.
FDA clears second bivalent COVID boosters for some AmericansSome Americans will soon be able to get another shot of the bivalent COVID-19 vaccines, the FDA authorized Tuesday, including seniors ages 65 and older and people with compromised immune systems.
Weiterlesen »
 FDA authorizes 2nd dose of updated Covid booster for older adultsPeople 65 and up can get a second dose of the updated booster at least four months after their last dose.
FDA authorizes 2nd dose of updated Covid booster for older adultsPeople 65 and up can get a second dose of the updated booster at least four months after their last dose.
Weiterlesen »
 Supreme Court to Decide Future of Abortion Pill MifepristoneWATCH: The FDA approved mifepristone initially some 20 years ago, but now the question is whether the FDA did it the right way, says legal analyst Dan Eaton.
Supreme Court to Decide Future of Abortion Pill MifepristoneWATCH: The FDA approved mifepristone initially some 20 years ago, but now the question is whether the FDA did it the right way, says legal analyst Dan Eaton.
Weiterlesen »
 Single Dose of Omicron-Targeting Vaccines to Become Main Covid-19 Shot in U.S.Americans seeking messenger RNA vaccines for Covid-19 for the first time will get one shot targeting both the Omicron variant and the original strain of the virus
Single Dose of Omicron-Targeting Vaccines to Become Main Covid-19 Shot in U.S.Americans seeking messenger RNA vaccines for Covid-19 for the first time will get one shot targeting both the Omicron variant and the original strain of the virus
Weiterlesen »