The emergency use authorization is for people 18 years of age and older determined to be at high risk for contracting monkeypox.
The U.S. Food and Drug Administration has issued an emergency use authorization for the JYNNEOS monkeypox vaccine.
Healthcare providers will be allowed to use the vaccine for intradermal injection for those 18-years-old and older who are determined to be at heightened risk of contracting monkeypox. FDA Commissioner Dr. Robert M. Califf said of vaccine supply, “In recent weeks the monkeypox virus has continued to spread at a rate that has made it clear our current vaccine supply will not meet the current demand,” he said. “The FDA quickly explored other scientifically appropriate options to facilitate access to the vaccine for all impacted individuals.
The JYNNEOS vaccine is administered beneath the skin in two doses administered 4 weeks apart. The EUA allows for a fraction of the dose to be administered in two doses four weeks apart, the FDA said. In June, the White House estimated that it would take at least $7 billion from Congress to fight the spread of monkeypox. Then in August Health and Human Services Secretary Xavier Becerra announced that the Biden administration had instructed his agency to"explore every option on the table" to fight the spread of the virus.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
FDA Authorizes Intradermal Use of Jynneos Vaccine for MonkeypoxFDA authorizes intradermal administration of the Jynneos vaccine for the treatment of monkeypox.
Weiterlesen »
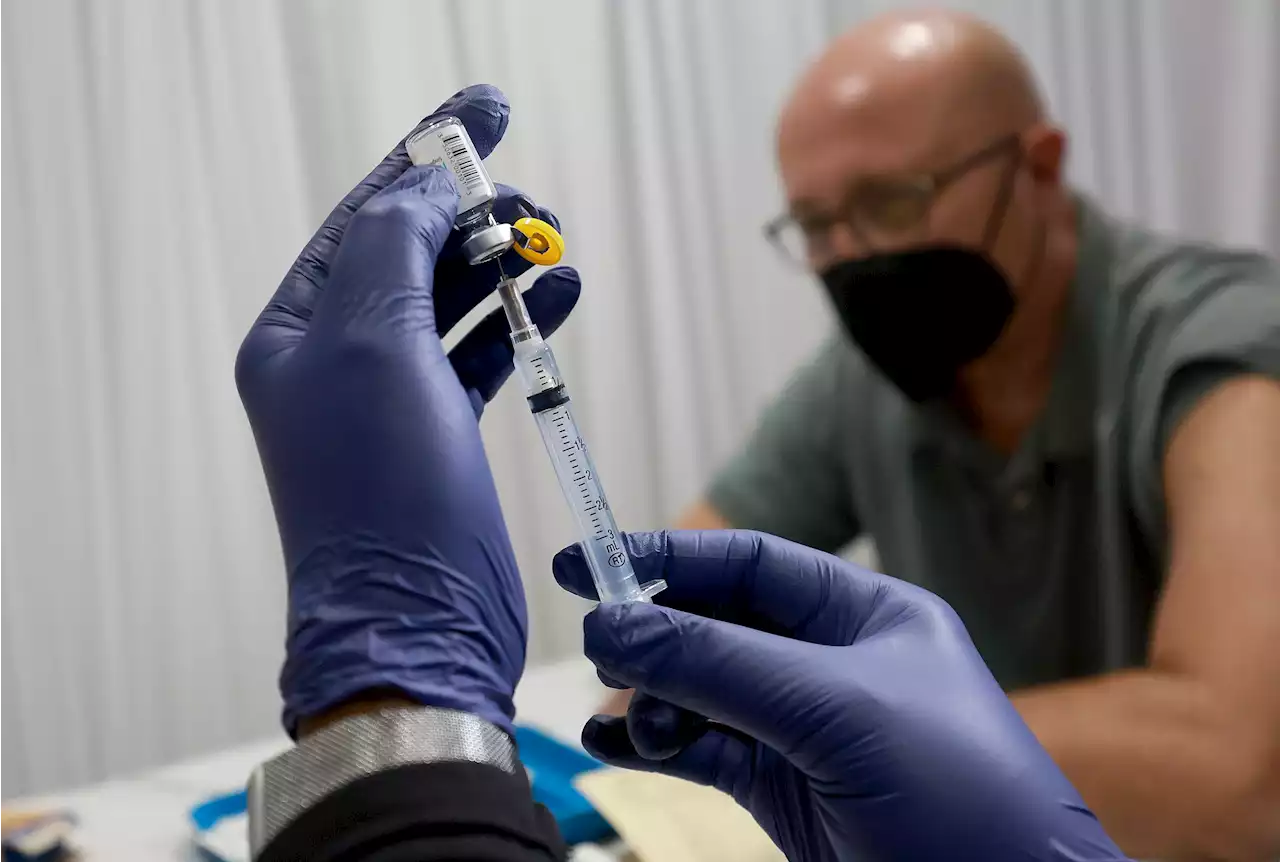 FDA Will Reportedly Change Dose Of Monkeypox Vaccine To Stretch SuppliesThe Biden administration plans to issue an emergency declaration that will allow healthcare workers to extract five doses from a one-dose vial of the Jynneos monkeypox vaccine by changing the method of administration, according to multiple outlets.
FDA Will Reportedly Change Dose Of Monkeypox Vaccine To Stretch SuppliesThe Biden administration plans to issue an emergency declaration that will allow healthcare workers to extract five doses from a one-dose vial of the Jynneos monkeypox vaccine by changing the method of administration, according to multiple outlets.
Weiterlesen »
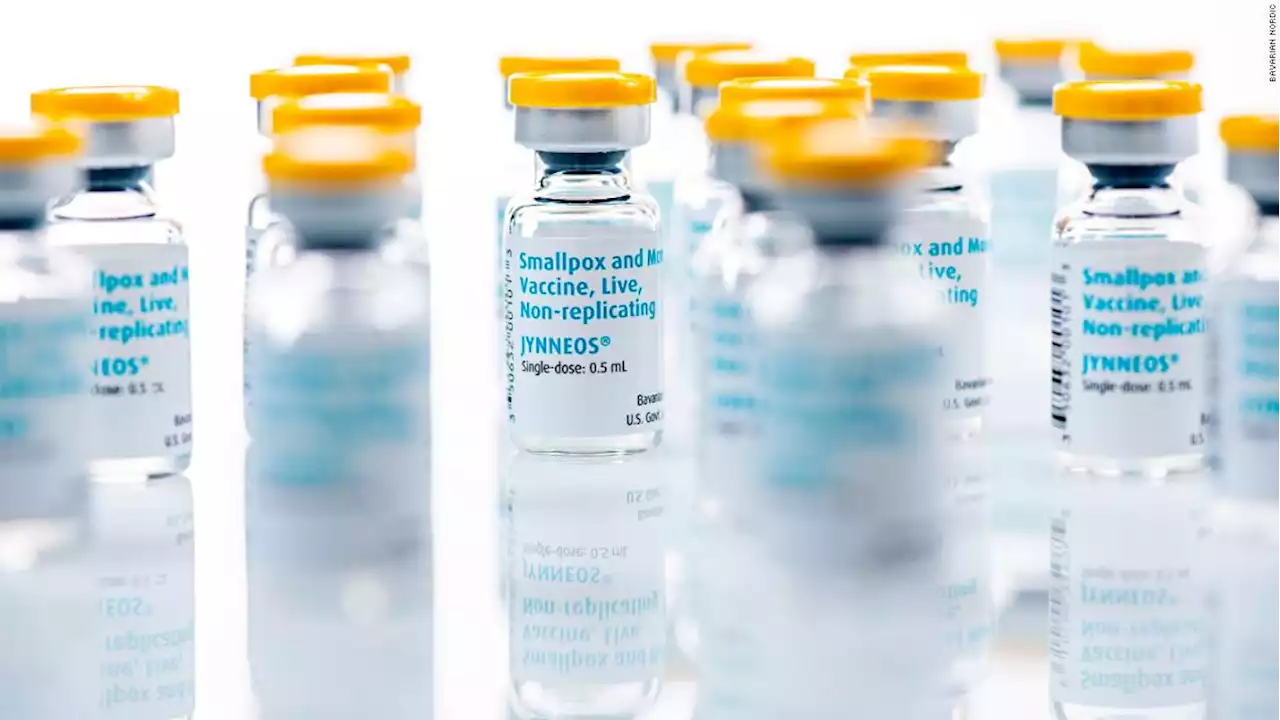 FDA authorizes change in how monkeypox vaccine is givenThe US Food and Drug Administration issued an emergency use authorization Tuesday that allows health-care providers to change how the Jynneos monkeypox vaccine is administered, stretching out the supply amid high demand.
FDA authorizes change in how monkeypox vaccine is givenThe US Food and Drug Administration issued an emergency use authorization Tuesday that allows health-care providers to change how the Jynneos monkeypox vaccine is administered, stretching out the supply amid high demand.
Weiterlesen »
 FDA expands monkeypox vaccine authorization to increase dose supply five-foldThe FDA will allow health-care providers to administer the shots through intradermal injection, or between the layers of the skin, for adults.
FDA expands monkeypox vaccine authorization to increase dose supply five-foldThe FDA will allow health-care providers to administer the shots through intradermal injection, or between the layers of the skin, for adults.
Weiterlesen »
 Protesters demand swifter action as FDA considers plans to split doses of monkeypox vaccineA local infectious disease expert on Monday said she supports the FDA's idea of splitting doses of the monkeypox vaccine to get the shots to more people.
Protesters demand swifter action as FDA considers plans to split doses of monkeypox vaccineA local infectious disease expert on Monday said she supports the FDA's idea of splitting doses of the monkeypox vaccine to get the shots to more people.
Weiterlesen »
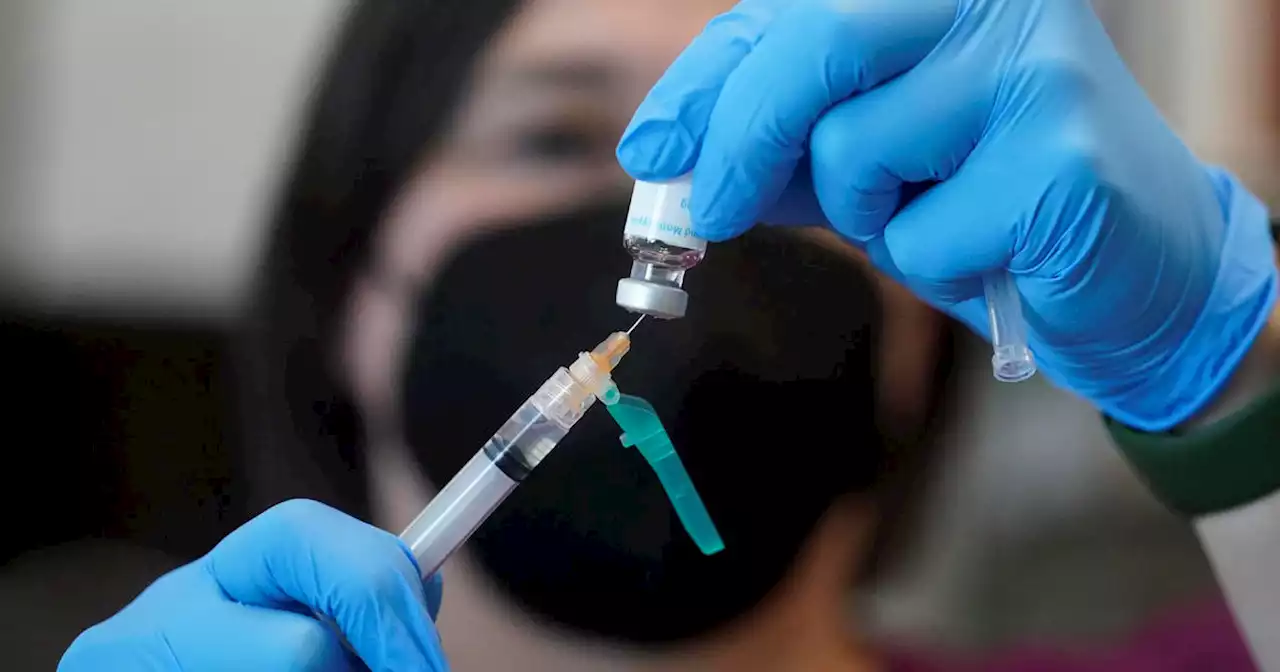 FDA issues emergency use authorization for new monkeypox vaccine protocolThere is a new protocol for monkeypox vaccines. On Tuesday, the FDA issued an emergency use authorization. It allows health care providers to change how the vaccine is administered. DaveCarlinTV reports.
FDA issues emergency use authorization for new monkeypox vaccine protocolThere is a new protocol for monkeypox vaccines. On Tuesday, the FDA issued an emergency use authorization. It allows health care providers to change how the vaccine is administered. DaveCarlinTV reports.
Weiterlesen »
