The White House expects vaccines to be available in pharmacies and doctor's offices by next week. TheBump Vaccines
Today, the US Food and Drug Administration authorized emergency use of the Moderna COVID-19 vaccine and the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in children as young as 6 months old. While FDA authorization is not the final step in the process must also issue its recommendation), according to a, vaccines could be available by next week at pharmacies, doctor’s offices and hospitals.
“Many parents, caregivers and clinicians have been waiting for a vaccine for younger children, and this action will help protect those down to 6 months of age. As we have seen with older age groups, we expect that the vaccines for younger children will provide protection from the most severe outcomes of COVID-19, such as hospitalization and death,” said FDA Commissioner Robert M. Califf, MD, in a.
The FDA conducted ongoing, randomized, blinded, placebo-controlled clinical trials in the United States and internationally to determine the safety and effectiveness of both Moderna and Pfizer vaccines. Over 2,800 children were involved in the study, ranging in age from 6 months to 5 years old. In both clinical trials, only mild side effects were observed, most commonly pain, redness and swelling at the injection site and fever.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
Weiterlesen »
 FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
Weiterlesen »
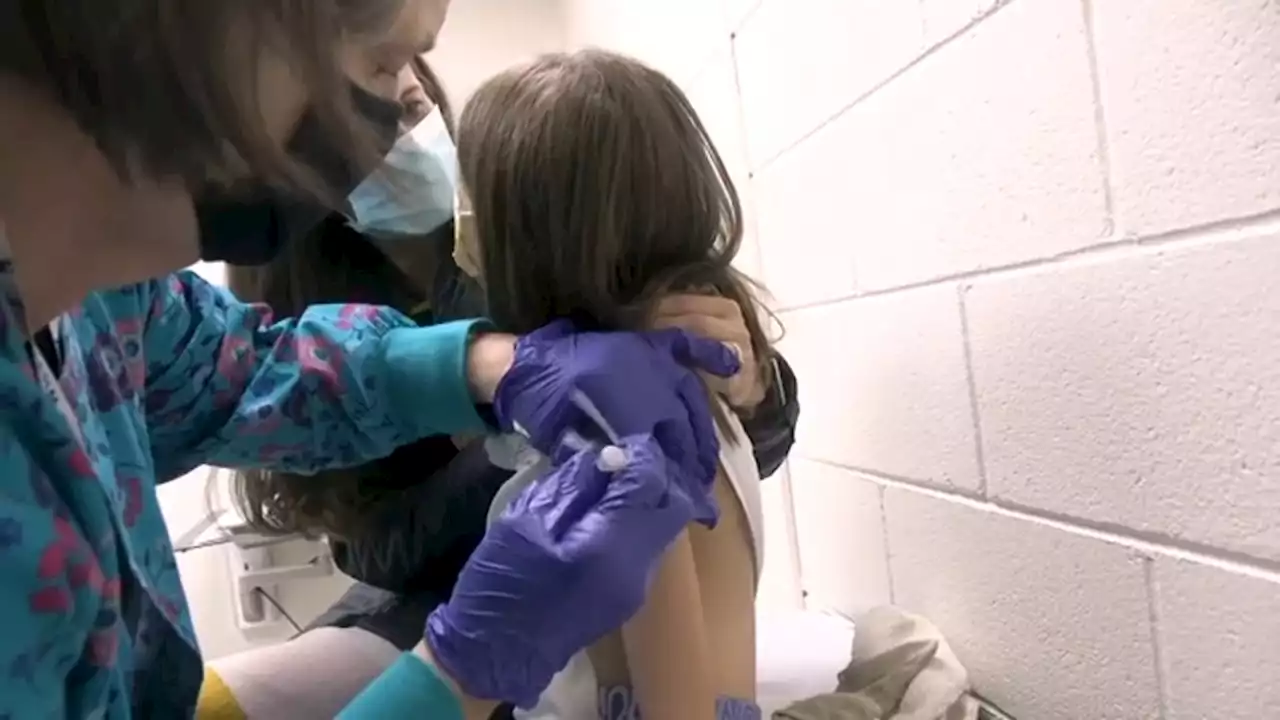 FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
Weiterlesen »
 FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
Weiterlesen »
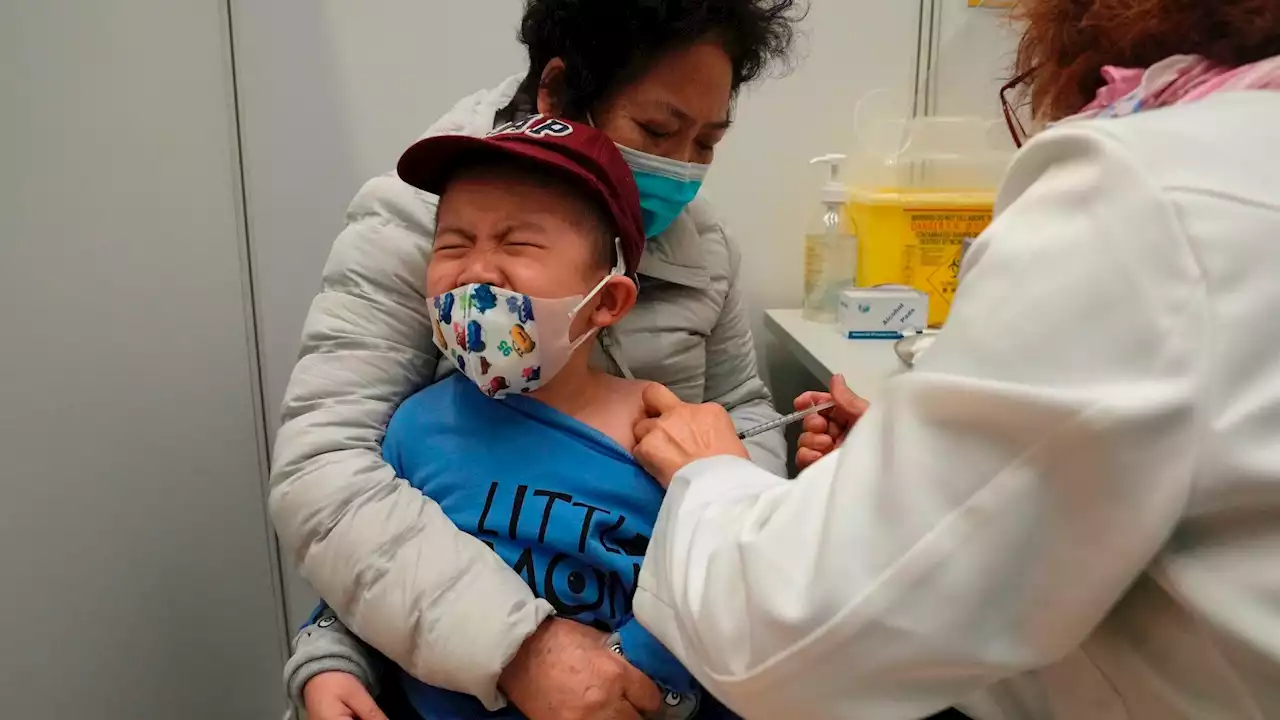 FDA Advisers Move COVID-19 Shots Closer for Kids Under 5The Food and Drug Administration’s vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
FDA Advisers Move COVID-19 Shots Closer for Kids Under 5The Food and Drug Administration’s vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
Weiterlesen »