FDAapproves abaloparatide (Tymlos) for increasing bone density in men with osteoporosis at high risk for fracture.
High risk for fracture is defined as a history of osteoporotic fracture or multiple risk factors for fracture. by the FDA in 2017 for the treatment of postmenopausal women at high risk for fracture due to a history of osteoporotic fracture or multiple fracture risk factors, or who hadn't responded to or were intolerant of other osteoporosis therapies.
The approval in men is based on results from the Abaloparatide for the Treatment of Men With Osteoporosis randomized, double-blind, placebo-controlled, phase 3 study,At the time, presenter Neil Binkley, MD, of the University of Wisconsin School of Medicine and Public Health Madison, told ,"Osteoporosis is underdiagnosed in men. Abaloparatide is another option for an ignored population."
Abaloparatide — a parathyroid-hormone–related protein analog — administered as a subcutaneous 80-μg injection daily for 12 months resulted in significant increases in bone mineral density at the lumbar spine, total hip, and femoral neck compared with placebo in men with osteoporosis, with no significant adverse effects, in ATOM.
In a company press release, Bruce Mitlak, MD, chief medical officer of Radius, said,"30% of all hip fractures occur in men and approximately 25% of men over the age of 50 years will sustain an osteoporotic-related fracture.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 U.S. FDA approves Roche's COVID-19 antibodyRoche Holding AG said on Wednesday the U.S. Food and Drug Administration (FDA) had approved its monoclonal antibody for treating COVID-19 in hospitalized adult patients.
U.S. FDA approves Roche's COVID-19 antibodyRoche Holding AG said on Wednesday the U.S. Food and Drug Administration (FDA) had approved its monoclonal antibody for treating COVID-19 in hospitalized adult patients.
Weiterlesen »
 Putin to Boost Russian Military, Signaling Protracted War in UkrainePresident Vladimir Putin approved an increase in Russia's military manpower while ordering the enhancement of its potential and capability, suggesting that the Kremlin is digging in for a protracted war effort
Putin to Boost Russian Military, Signaling Protracted War in UkrainePresident Vladimir Putin approved an increase in Russia's military manpower while ordering the enhancement of its potential and capability, suggesting that the Kremlin is digging in for a protracted war effort
Weiterlesen »
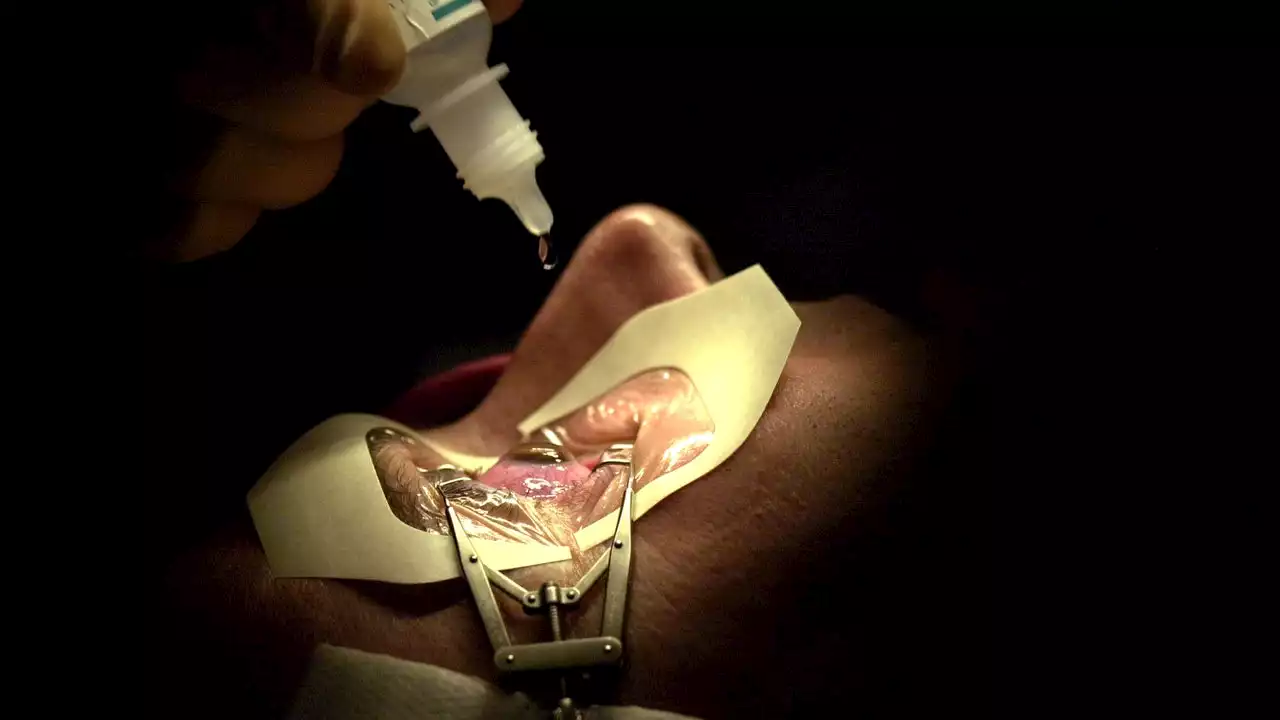 FDA weighs new guidance about risks of LASIK eye surgeryThe FDA wants LASIK patients to know more about the risks of the popular eye surgery, but doctors say the agency
FDA weighs new guidance about risks of LASIK eye surgeryThe FDA wants LASIK patients to know more about the risks of the popular eye surgery, but doctors say the agency
Weiterlesen »
 Horse food recalled after 45 deaths, FDA saysA snack for horses is being recalled after 45 horses died from eating it, according to the FDA.
Horse food recalled after 45 deaths, FDA saysA snack for horses is being recalled after 45 horses died from eating it, according to the FDA.
Weiterlesen »
 FDA Commissioner Urges Parents Not to Stockpile Children's Flu Medications Amid ShortagesThe surge in flu cases and COVID-19 infections, along with elevated levels of RSV, has caused elevated demand for children's over-the-counter cold and flu medications — but the FDA commissioner is urging parents to resist buying more than what they need
FDA Commissioner Urges Parents Not to Stockpile Children's Flu Medications Amid ShortagesThe surge in flu cases and COVID-19 infections, along with elevated levels of RSV, has caused elevated demand for children's over-the-counter cold and flu medications — but the FDA commissioner is urging parents to resist buying more than what they need
Weiterlesen »