The World Health Organization has raised unspecified concerns about the manufacturing of Covaxin, one of India’s home-grown COVID19 vaccines.
According to a WHO spokesperson, ANVISA later told the agency that Bharat Biotech had addressed the deficiencies, and in November 2021, WHO awarded the vaccine an emergency use listing. The listing is a prerequisite for a vaccine to be supplied through the COVID-19 Vaccines Global Access Facility, an effort by WHO and two other organizations to provide doses to low- and middle-income countries, and a stamp of approval that helps member countries decide which vaccines to deploy.
But when WHO inspectors visited the plant between 14 and 21 March, they found several GMP deficiencies, some of which overlapped with those identified by ANVISA, according to the spokesperson. The company had changed its manufacturing process after its listing but hadn’t communicated these changes to CDSCO and WHO for evaluation and validation.
Hans Meerburg, a Netherlands-based vaccine-quality consultant, says manufacturers are required to inform drug regulators of any major postapproval changes, because they can impact the safety, efficacy, or quality of the vaccine. “If not, the product may not comply with specifications, such as potency, or the absence of active material,” Meerburg says.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 Dallas-based Vaxxinity begins Phase 3 trial for COVID-19 booster vaccineIn the world of vaccine research, starting a new vaccine candidate in the early stages of the long and expensive development process is relatively commonplace. ...
Dallas-based Vaxxinity begins Phase 3 trial for COVID-19 booster vaccineIn the world of vaccine research, starting a new vaccine candidate in the early stages of the long and expensive development process is relatively commonplace. ...
Weiterlesen »
 San Diego will be part of national clinical trial on COVID-19 vaccinesEffort will be led locally by UC San Diego with almost half of participants 65 or older
San Diego will be part of national clinical trial on COVID-19 vaccinesEffort will be led locally by UC San Diego with almost half of participants 65 or older
Weiterlesen »
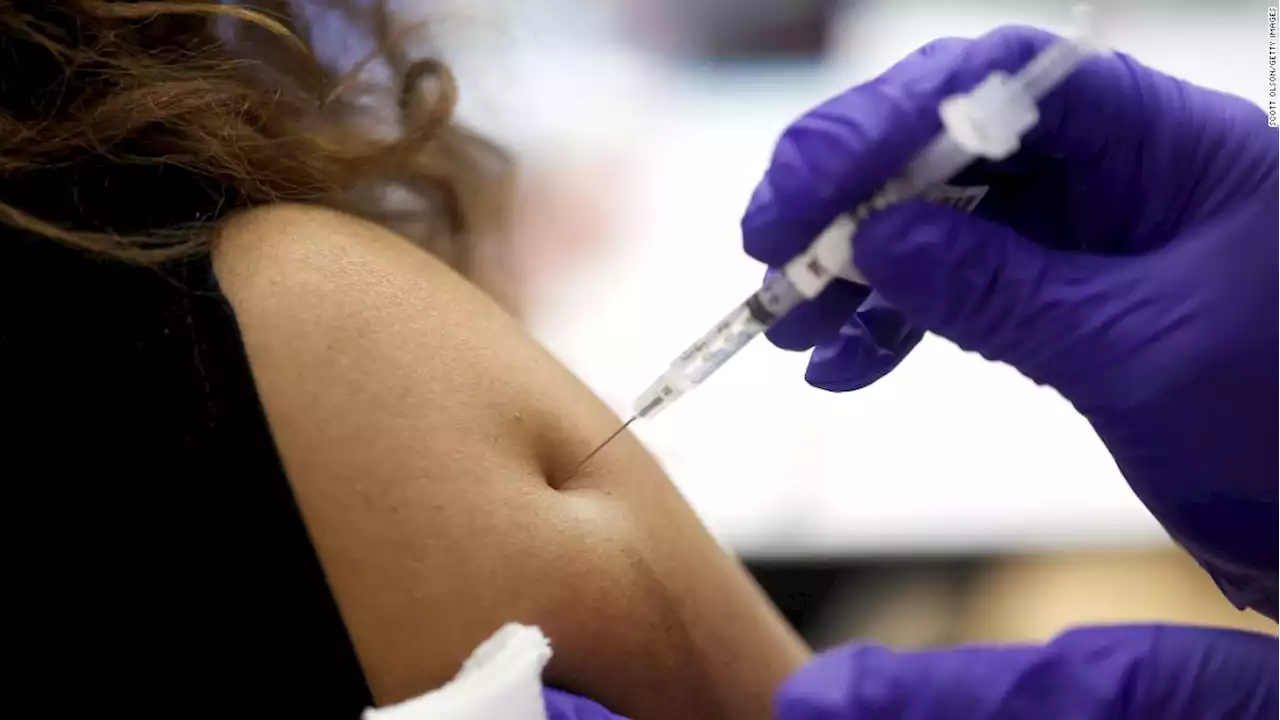 Protection against infection offered by fourth Covid-19 vaccine dose wanes quickly, Israeli study findsA fourth dose of the Pfizer/BioNTech Covid-19 vaccine seems to offer short-lived protection against infection overall, but protection against severe illness did not wane for at least several weeks, according to a new study.
Protection against infection offered by fourth Covid-19 vaccine dose wanes quickly, Israeli study findsA fourth dose of the Pfizer/BioNTech Covid-19 vaccine seems to offer short-lived protection against infection overall, but protection against severe illness did not wane for at least several weeks, according to a new study.
Weiterlesen »
 Study finds Trump may have swayed some COVID-19 vaccine skeptics - The San Francisco ExaminerResearchers from UC Berkeley and Stanford found an uptick in vaccination rates amongst right leaning counties that viewed a 27-second advertisement showcasing Trump’s support for the vaccine.
Study finds Trump may have swayed some COVID-19 vaccine skeptics - The San Francisco ExaminerResearchers from UC Berkeley and Stanford found an uptick in vaccination rates amongst right leaning counties that viewed a 27-second advertisement showcasing Trump’s support for the vaccine.
Weiterlesen »
 WHO finds potential link between COVID-19 vaccine and hearing issuesThe World Health Organization is now investigating hearing loss and ringing in the ears as potential rare side effects of the COVID-19 vaccines: abc15
WHO finds potential link between COVID-19 vaccine and hearing issuesThe World Health Organization is now investigating hearing loss and ringing in the ears as potential rare side effects of the COVID-19 vaccines: abc15
Weiterlesen »
COVID vaccines: head-to-head comparison reveals how they stack upComparison of the immune response to four prominent COVID-19 vaccines is among the most thorough so far, authors say.
Weiterlesen »