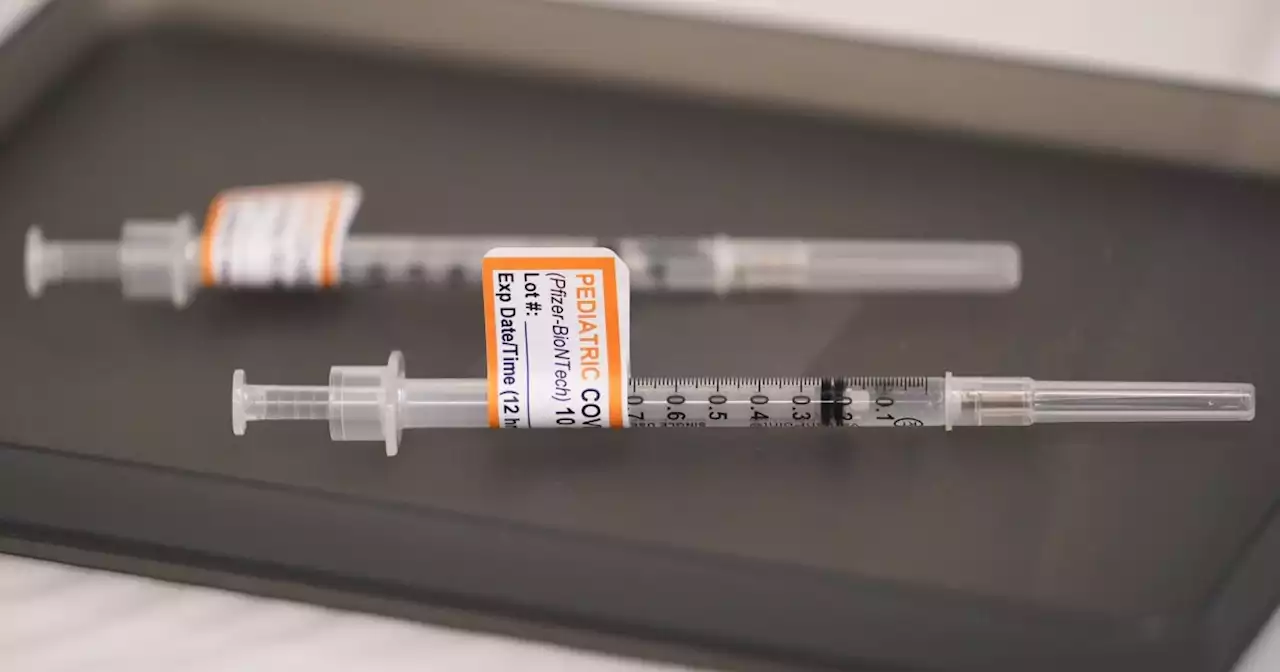
The Food and Drug Administration on Wednesday authorized the bivalent COVID-19 booster shot for younger children.
Centers for Disease Control Director Dr. Rochelle Walensky signed off on the Food and Drug Administration's authorization of the bivalent COVID-19 booster shot for children as young as five years old.
The bivalent vaccine targets the omicron variant, which continues to be the dominant strain across the U.S.The Pfizer booster can be administered at least two months following completion of the primary vaccine series in children five years and older."Updated COVID-19 vaccines add Omicron BA.4 and BA.
Just over 30% of children as young as five have completed their primary vaccine series, the CDC reports. Wednesday's authorization comes about three months after the FDA authorized the bivalent booster for people for adults.
Österreich Neuesten Nachrichten, Österreich Schlagzeilen
Similar News:Sie können auch ähnliche Nachrichten wie diese lesen, die wir aus anderen Nachrichtenquellen gesammelt haben.
 FDA authorizes updated bivalent COVID-19 booster for children aged 5 and olderThe Food and Drug Administration authorized the updated bivalent COVID-19 booster for children ages 5 and older Monday.
FDA authorizes updated bivalent COVID-19 booster for children aged 5 and olderThe Food and Drug Administration authorized the updated bivalent COVID-19 booster for children ages 5 and older Monday.
Weiterlesen »
 FDA authorizes omicron-targeting COVID-19 booster for younger kidsThe Food and Drug Administration authorized the bivalent COVID-19 booster shot for younger children. Moderna's booster is authorized for children six years and older.
FDA authorizes omicron-targeting COVID-19 booster for younger kidsThe Food and Drug Administration authorized the bivalent COVID-19 booster shot for younger children. Moderna's booster is authorized for children six years and older.
Weiterlesen »
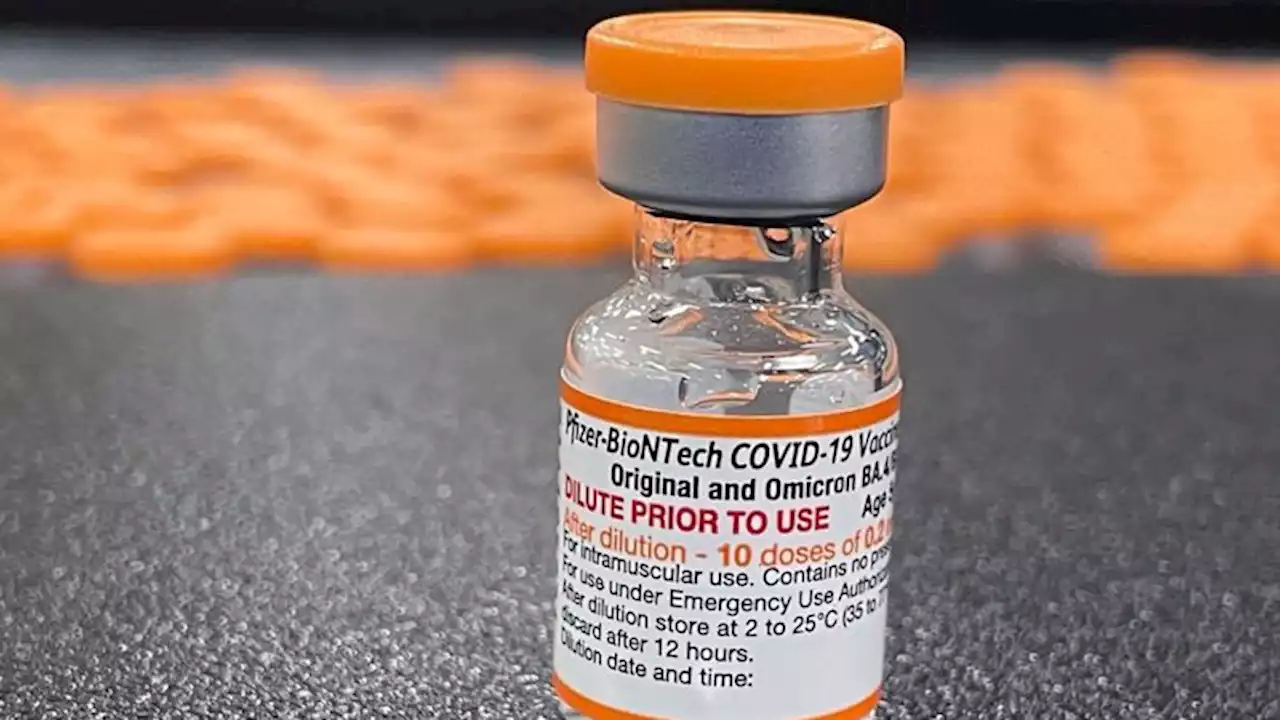 FDA authorizes updated Covid-19 booster shots for children as young as 5 | CNNFDA authorizes updated Covid-19 booster shots for children as young as 5. The boosters target the original coronavirus strain and Omicron subvariants.
FDA authorizes updated Covid-19 booster shots for children as young as 5 | CNNFDA authorizes updated Covid-19 booster shots for children as young as 5. The boosters target the original coronavirus strain and Omicron subvariants.
Weiterlesen »
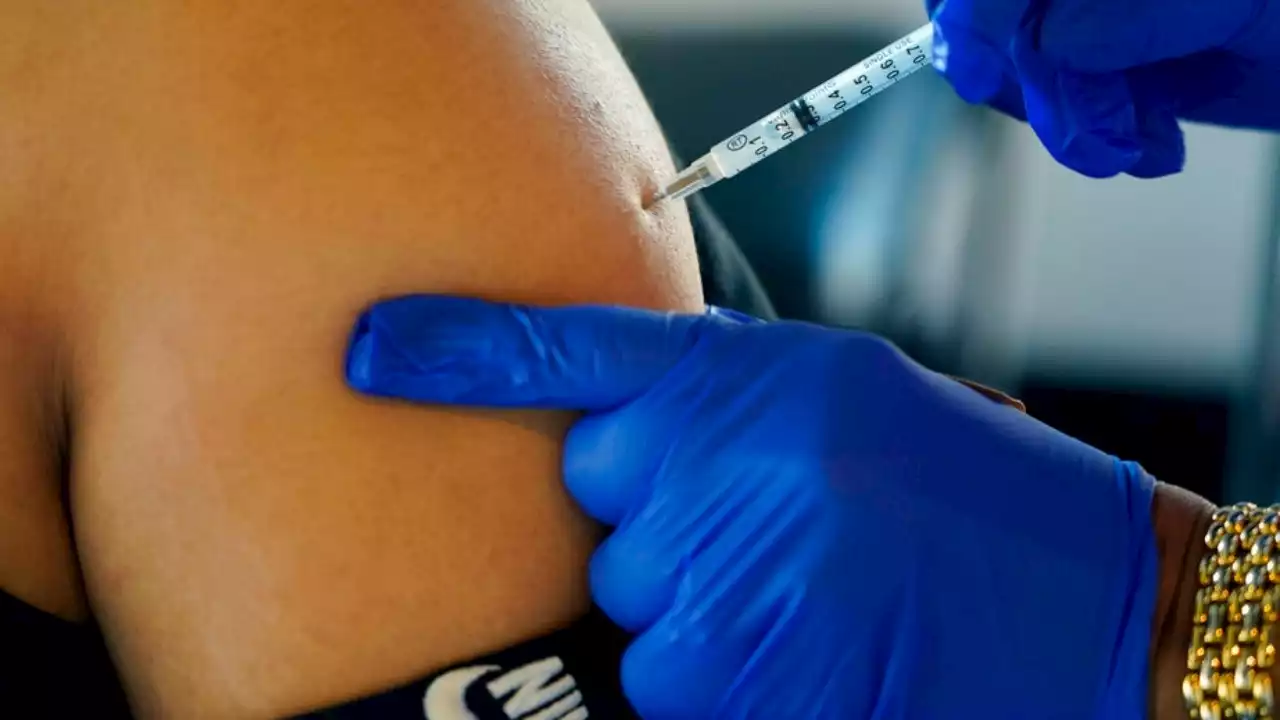 FDA authorizes bivalent COVID boosters for young childrenThe Food and Drug Administration announced Wednesday that it was amending is emergency use authorization of bivalent COVID vaccines for use in younger kids.
FDA authorizes bivalent COVID boosters for young childrenThe Food and Drug Administration announced Wednesday that it was amending is emergency use authorization of bivalent COVID vaccines for use in younger kids.
Weiterlesen »
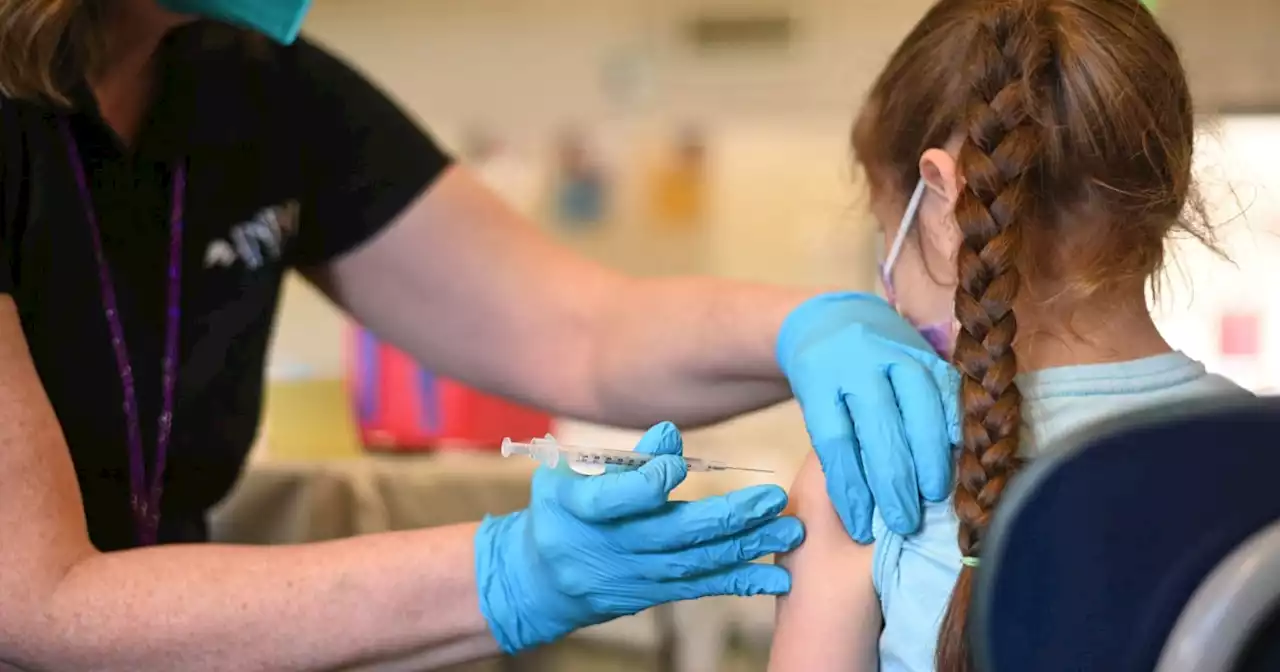 Children as young as 5 can now get omicron booster shots, CDC saysCDC Director Dr. Rochelle Walensky signed off on the updated Covid vaccines only hours after authorization from the Food and Drug Administration.
Children as young as 5 can now get omicron booster shots, CDC saysCDC Director Dr. Rochelle Walensky signed off on the updated Covid vaccines only hours after authorization from the Food and Drug Administration.
Weiterlesen »
 FDA authorizes updated COVID-19 booster for younger childrenThe Food and Drug Administration authorized the updated bivalent COVID-19 booster for children ages 5 and older Monday.
FDA authorizes updated COVID-19 booster for younger childrenThe Food and Drug Administration authorized the updated bivalent COVID-19 booster for children ages 5 and older Monday.
Weiterlesen »